You have no items in your shopping cart
Aluminum sulfate hydrate 99+%
- Buy 2 and save 5%
What is Aluminum Sulphate?
Aluminum sulfate is the chemical compound with formula Al2(SO4)3, where aluminum has oxidation number +3. Under standard conditions it is a white crystalline solid, soluble in water and insoluble in alcohol. It exists in anhydrous form and in various hydrated forms. It is one of the most important products of industrial inorganic chemistry. It is mostly used in the paper industry, but has several other uses.
Al2(SO4)3 in anhydrous form is unusual, but occurs naturally in the rare mineral millosevichite, which can be found near volcanoes or after burning mining waste in landfills. There are also several hydrated forms; the most common are Al2(SO4)3 • 14H2O and Al2(SO4)3 • 18H2O. The form Al2(SO4)3 • 17H2O occurs naturally in the mineral alunogen.
What is aluminum sulfate used for?
Al2 (SO4) 3 is used in multiple applications:
About two-thirds of the production is used in the paper industry, where it is used to regulate the pH of wood pulp residues, to treat process water, to treat cellulosic fibers and to prevent the ink from spreading.
In water treatment, it serves as a flocculant to capture impurities, adjust pH and remove phosphates and bacteria.
When dyeing fabrics, it serves as a mordant, facilitating the adhesion of dyes to the fibers. It is also used as a stabilizer of diazo solutions and as a neutralizer of alkalis in naphthol dyeing.
In the construction industry, it is used as a waterproofing agent and accelerator of concrete setting.
As a catalyst and for the preparation of other chemicals.
To lower the pH of soils, as it hydrolyzes to form insoluble aluminum hydroxide, releasing a dilute sulfuric acid solution. For example, it is used to obtain the blue flowers of hydrangeas.
Buy aluminum sulfate?
You can buy aluminum sulfate at Laboratoriumdiscounter. Why? Because Laboratoriumdiscounter offers the best quality for a friendly price. Shipped fast!
Technical data:
Aluminum Sulfate Hydrate
Empirical formula Al2(SO4)3 X H2O
Density (D) 0.6-0.8 g/cm³
CAS no. 16828-12-9
Downloads
$$$$$
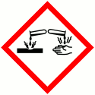
Hazard statements
H318 Causes serious eye damage
Precautionary statements
Precautionary statements - prevention
P280 Wear protective gloves/eye protection
Precautionary statements - response
P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact
lenses, if present and easy to do. Continue rinsing
P310 Immediately call a POISON CENTER/doctor