You have no items in your shopping cart
Calcium chloride 96% anhydrous, pure
- Buy 2 and save 5%
- Buy 6 and save 15%
What is Calcium Chloride?
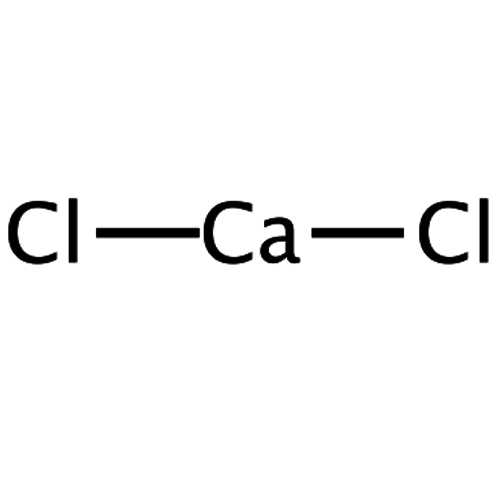
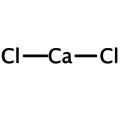
Calcium chloride is a chloride of the alkaline earth metal calcium with the formula CaCl2. Calcium is in the oxidation state +2, chlorine has the oxidation state -1.
What is calcium chloride used for?
Due to its hygroscopicity, anhydrous calcium chloride is an important drying agent in the laboratory, for example in the desiccator, and in technical chemistry for gases and liquids.
It is also used as a road salt (de-icing agent) and as a hexahydrate for the production of cold mixes.
Areas of application in the construction industry are drying living spaces, use as an antifreeze, especially as an antifreeze and setting accelerator in concrete, and as a dust binder (e.g. on construction sites and as a filler for blasting work). The use of calcium chloride as a setting accelerator in concrete was banned in Germany in 1963 due to the corrosive effects of chloride on the iron reinforcement.
Calcium chloride is added to the fill water of concrete swimming pools to increase the water hardness and reduce the erosion of concrete by dissolving calcium compounds from the concrete according to the principle of least limitation.
In addition to antifreeze, most calcium chloride is used to bind sand and dust on unpaved roads. Being highly hygroscopic, a concentrated calcium chloride solution applied to the road attracts moisture and suppresses the removal of road dust. The road surface needs to be leveled less often and the surface needs to be renewed less often.
The food industry uses it as a complexing agent, flavor enhancer and stabilizer (including in drinking water treatment). It is approved in the EU as a food additive with the number E 509. The daily intake is believed to be 160-345 mg (note that this specific calcium chloride is not food grade).
It is added to canned vegetables and sliced fruits as a firming agent. It is also used to coagulate proteins in food production, for example in the production of cheese, tofu or artificial caviar. In cheese making, calcium chloride is sometimes added to milk to improve the properties of the precipitated casein.
It is used as an electrolyte in sports drinks.
Because of its salty taste, it can replace table salt in pickled cucumbers and pickled vegetables.
Calcium chloride is used in beer brewing to adjust the mineral content of the brewing water and influence flavor and yeast growth.
Calcium chloride is used to treat the surface of fruits. Apples are treated in the late growth stage to prevent the deficiency spot and other disorders.
Taking advantage of the exothermic hydration in the reaction with water, calcium chloride is used to heat ready-to-drink drinks.
In molecular biology, it is used to produce competent cells. Calcium ions change the permeability of the cell membrane thus increasing the cell's absorptive capacity for DNA.
Calcium chloride is used in marine aquariums to increase calcium levels.
Buy Calcium Chloride CAS 10043-52-4 ?
Buy high quality calcium chloride at a low price at Laboratoriumdiscounter.nl. Always a volume discount when purchasing multiple packages and delivered quickly.
Technical data
Empirical formula CaCl2
Molar mass (M) 110.99 g/mol
Density (D) 2.15 g/cm³
Boiling point (bp) 1935 °C
Melting point (mp) 775°C
WCK 1
CAS no. [10043-52-4]
EC-No. 233-140-8
$$$$$
Hazard Statements
H319 Causes serious eye irritation
Safety Recommendations
Precautions - prevention
P280 Wear protective gloves/eye protection.
Precautions - response
P305+P351+P338 IF IN EYES: Rinse cautiously with water for an
amount of minutes; remove contact lenses, if possible; keep rinsing.
P337+P313 If eye irritation persists: Get medical advice/attention.