You have no items in your shopping cart
Calcium hydroxide ≥97 %, Ph.Eur., USP, BP, Foodgrade E526
- Buy 2 and save 5%
- Buy 6 and save 10%
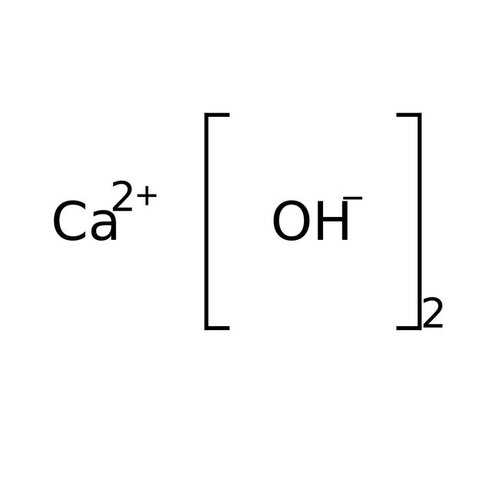
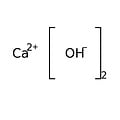
Calcium hydroxide is an inorganic chemical, an ionic compound of the calcium cation and the hydroxide anion, with the formula Ca (OH) 2. This ancient alkali is also called "slaked lime", "fatty lime" and "air lime" because this corrosive and hygroscopic powder was prepared from quicklime or calcium oxide produced in the past by the lime kiln from lime. It is also a rare natural mineral, density 2.23, called "portlandite" by mineralogists because it is a simple product of Portland cement hydrolysis.
Applications
-Industry
Metallurgical: In the production of magnesium, two kinds of production process can be used: electrolytic process or thermal reduction process, hydrated lime is used in the electrolytic process.
Chemistry: in pesticide mixtures; in the process for the neutralization of excess acid, in the oil industry; in the manufacture of crude oil additives; in the petrochemical industry for the production of solid oil; in the manufacture of
calcium stearate; as a filler and as a raw material for obtaining precipitated calcium carbonate (CCP or PCC).
Food industries:
Sugar industry (especially in cane sugar)
Ostreiculture
Fish farming
Dairy industry
Manufacture of glues and jellies
Preservation of Fruits and Vegetables: To remove excess CO2 in the Controlled Atmosphere (CA) chambers before
preserving fruit and vegetables (including flowers)
Wheat and maize treatment: Component for the nixtamalization of maize to produce tortillas.
Salt production: to rid a brine of calcium and magnesium carbonates in the production of table salt.
For processing water for alcoholic and carbonated drinks
Pharmacopoeia
Cosmetics
Paper industry 6
Brake disc manufacturing
Ebonite production
Dental and dental equipment: Root canal treatment equipment Endodontics or direct or indirect pulp protection in dental coronary restorations [dental surgery]
-Construction
Hydraulic lime is used in construction, which is mixed with about 20% clay so that it hardens in the same way as cement.
Infrastructures: in soil stabilization to improve the properties of clay soils and in hot bituminous mixtures to increase their durability.
Construction: In lime mortars, 8 whitewashed paints (for the preparation of dry mixes for painting and decorating, and as paint for many
sports fields such as football and tennis), stucco and precast lime (artificial limestone and compressed earth blocks).
-Environmental protection
Drinking Water Treatment (Purification): It is used to improve the quality of the water consumed by humans, to soften, purify, eliminate turbidity, neutralize acidity, and remove silica and other impurities.
Wastewater Treatment: Lime is very commonly used in the conventional chemical treatment of industrial wastewater, essentially of an inorganic nature.
Lime is a readily available alkali, widely used in the treatment or line of sludge in urban wastewater treatment plants or in industrial water of an organic nature.
Remineralization of desalinated water: By adding lime, the desalinated water can be conditioned, which can vary from pH adjustment and reduction of aggressiveness to remineralization of the water through the contribution of calcium. Lime is essential for the eventual treatment of the water by seawater desalination, as it contains one of the basic nutrients - calcium - and is necessary for maintaining the lime-carbon balance to prevent deposits or corrosion.
Gas Purification: Depending on the process, lime is the most profitable and natural desulphurizer that removes sulfur dioxide and other acidic gases (HCl, HF and NOx) from industrial vapors from household waste incinerators, thermal power plants and industry in general. Lime is also used to remove persistent organic compounds (POPs) such as dioxins and furans and heavy metals from municipal and industrial incinerators.
Waste Treatment: Lime, in addition to being an integral part of various chemical treatments, is used as a means of preventing bad odors and water contamination from leaching.
Treatment of contaminated soils: The techniques used in the treatment of contaminated soils are grouped as follows:
Physicochemicals
Stabilization - coagulation
Biological
Thermal
In the physico-chemical treatment or method (which is a process of converting the waste by adding a series of chemical compounds to achieve the desired purpose), lime is used in the techniques of neutralization, precipitation and dechlorination. Regarding the stabilization / coagulation technique (the main purpose of which is to reduce the mobility and solubility of pollutants present in the soil, reduce their toxicity and eliminate their leaching), there is a variant called "Coagulation with lime and pozzolanic. materials ".
-Agriculture
The applications in agriculture are:
Amendment: it is used to improve the properties of agricultural soils: soil acidity, porosity and biological activity.
Fertilizer: Provides calcium, a nutrient for plants.
Compost: It is used to obtain compost from agricultural, agro-industrial and municipal waste.
Biocide: It can be used as a biocide for the purpose of destroying, counteracting, neutralizing, preventing the action or exercising control of another type on any organism harmful by chemical or biological means.
Phytosanitary Treatments: It is used in the preparation of Bordeaux mixture to neutralize the acidic pH of the copper sulfate solution, which would cause burns to the leaves and stems of the plants. Bordeaux mixture is a fungicide used to prevent or cure fungal diseases such as vine, scab or speckled pear and apple trees, peach tree lepra, etc.
Animal feed: Due to its high reaction rate, lime is used as a reagent for the production of calcium soaps for the production of additives and derivatives of animal feed.
In addition, lime is used in acidic soils (raising the pH and supplying calcium as a nutrient), which changes the composition of the pastures, allowing the emergence of legumes that are better digestible for livestock and have a higher protein content. This operation in acidic soils will cause a range of species to emerge in its floristic composition, including alfalfa, recognized by most farmers as the queen of fodder crops.
Calcium Hydroxide is a food additive (E5269) that is used as a pH regulator for foods and as a stabilizer / stabilizer10 in many products.
Technical data:
Empirical formula Ca (OH) 2
Molar mass (M) 74.10 g / mol
Density 2.24
Melting point (mp) 550 ° C (dec.)
Solubility: 1.7 g / l (H2O, 20 ° C)
WGK 1
CAS No. [1305-62-0]
EC no. 215-137-3
Downloads
$$$$$
Hazard statements
H315 Causes skin irritation
H318 Causes serious eye damage
H335 May cause respiratory irritation
Safety recommendations
Precautions - prevention
P260 Do not breathe dust.
P280 Wear protective gloves / eye protection.
Precautions - response
P302 + P352 IF ON SKIN: Wash with plenty of water.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P310 Immediately call a POISON CENTER / doctor