You have no items in your shopping cart
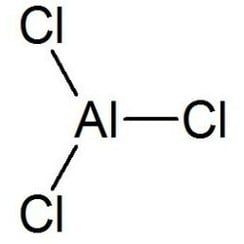
Aluminium chloride
Aluminium chloride (AlCl3), also known as aluminium trichloride, is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron(III) chloride, giving it a yellow color. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature.
Uses
Anhydrous aluminium trichloride
AlCl3 is probably the most commonly used Lewis acid and also one of the most powerful. It finds application in the chemical industry as a catalyst for Friedel–Crafts reactions, both acylations and alkylations. Important products are detergents and ethylbenzene. It also finds use in polymerization and isomerization reactions of hydrocarbons.
The Friedel–Crafts reaction is the major use for aluminium chloride, for example in the preparation of anthraquinone (for the dyestuffs industry) from benzene and phosgene. In the general Friedel–Crafts reaction, an acyl chloride or alkyl halide reacts with an aromatic system as shown:
The alkylation reaction is more widely used than the acylation reaction, although its practice is more technically demanding because the reaction is more sluggish. For both reactions, the aluminium chloride, as well as other materials and the equipment, should be dry, although a trace of moisture is necessary for the reaction to proceed. A general problem with the Friedel–Crafts reaction is that the aluminium chloride catalyst sometimes is required in full stoichiometric quantities, because it complexes strongly with the products. This complication sometimes generates a large amount of corrosive waste. For these and similar reasons, more recyclable or environmentally benign catalysts have been sought. Thus, the use of aluminium chloride in some applications is being displaced by zeolites.
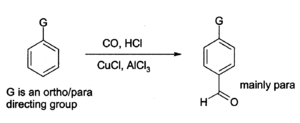
Aluminium chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gattermann-Koch reaction which uses carbon monoxide, hydrogen chloride and a copper(I) chloride co-catalyst.
Aluminium chloride finds a wide variety of other applications in organic chemistry. For example, it can catalyse the "ene reaction", such as the addition of 3-buten-2-one (methyl vinyl ketone) to carvone:
AlCl3 is also widely used for polymerization and isomerization reactions of hydrocarbons. Important examples include the manufacture of ethylbenzene, which used to make styrene and thus polystyrene, and also production of dodecylbenzene, which is used for making detergents.
Aluminium chloride combined with aluminium in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g. bis(benzene)chromium, from certain metal halides via the so-called Fischer-Hafner synthesis.
Hydrated aluminium chlorides
The dihydrate has few applications, but aluminium chlorohydrate is a common component in antiperspirants at low concentrations. Hyperhidrosis sufferers need a much higher concentration (12% or higher), sold under such brand names as Driclor.