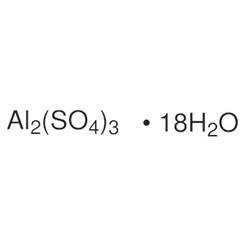You have no items in your shopping cart
Aluminium sulfate
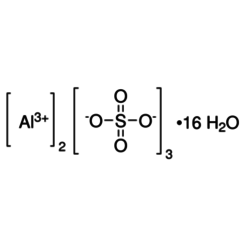
Aluminium sulfate is a chemical compound with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and waste water treatment plants, and also in paper manufacturing.
The anhydrous form occurs naturally as a rare mineral millosevichite, found e.g. in volcanic environments and on burning coal-mining waste dumps. Aluminium sulfate is rarely, if ever, encountered as the anhydrous salt. It forms a number of different hydrates, of which the hexadecahydrate Al2(SO4)3•16H2O and octadecahydrate Al2(SO4)3•18H2O are the most common. The heptadecahydrate, whose formula can be written as [Al(H2O)6]2(SO4)3•5H2O, occurs naturally as the mineral alunogen.
Aluminium sulfate is sometimes called alum or papermaker's alum in certain industries. However, the name "alum" is more commonly and properly used for any double sulfate salt with the generic formula XAl(SO4)2·12H
2O, where X is a monovalent cation such as potassium or ammonium.