You have no items in your shopping cart
Ammonium bicarbonate
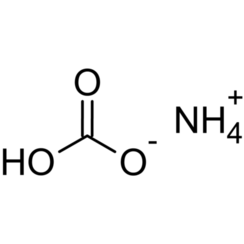
Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3, simplified to NH5CO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to carbon dioxide, water and ammonia.
Ammonium bicarbonate is used in the food industry as a leavening agent for flat baked goods, such as cookies and crackers. It was commonly used in the home before modern-day baking powder was made available. Many baking cookbooks, especially from Scandinavian countries, may still refer to it as hartshorn or hornsalt, while it is known as "hirvensarvisuola" in Finnish, "hjortetakksalt" in Norwegian, "hjortetakssalt" in Danish, "hjorthornssalt" in Swedish, and "Hirschhornsalz" in German (lit., "salt of hart's horn"). Although there is a slight smell of ammonia during baking, this quickly dissipates, leaving no taste. It is used in, for example, Swedish "drömmar" biscuits and Danish "brunkager" Christmas biscuits, and German Lebkuchen. In many cases it may be replaced with baking soda or baking powder, or a combination of both, depending on the recipe composition and leavening requirements. Compared to baking soda or potash, hartshorn has the advantage of producing more gas for the same amount of agent, and of not leaving any salty or soapy taste in the finished product, as it completely decomposes into water and gaseous products that evaporate during baking. It cannot be used for moist, bulky baked goods however, such as normal bread or cakes, since some ammonia will be trapped inside and will cause an unpleasant taste. It has been assigned E number E503 for use as a food additive in the European Union.
It is commonly used as an inexpensive nitrogen fertilizer in China, but is now being phased out in favor of urea for quality and stability. This compound is used as a component in the production of fire-extinguishing compounds, pharmaceuticals, dyes, pigments, and it is also a basic fertilizer, being a source of ammonia. Ammonium bicarbonate is still widely used in the plastics and rubber industry, in the manufacture of ceramics, in chrome leather tanning, and for the synthesis of catalysts.[citation needed]
It is also used for buffering solutions to make them slightly alkaline during chemical purification, such as high-performance liquid chromatography. Because it entirely decomposes to volatile compounds, this allows rapid recovery of the compound of interest by freeze-drying.
Ammonium bicarbonate is also a key component of the expectorant cough syrup "Senega and Ammonia".