You have no items in your shopping cart
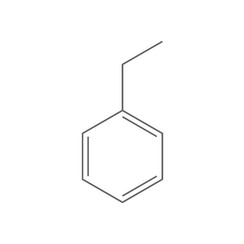
Ethylbenzene
Ethylbenzene is an organic compound with the formula C6H5CH2CH3. It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene, the precursor to polystyrene, a common plastic material. In 2012, more than 99% of ethylbenzene produced was consumed in the production of styrene.
Occurrence and applications
Ethylbenzene occurs naturally in coal tar and petroleum.
The dominant application of ethylbenzene is role as an intermediate in the production of polystyrene. Catalytic dehydrogenation of ethylbenzene gives hydrogen and styrene:
- C
6H
5CH
2CH
3 → C6H5CH=CH2 + H
2
As of May 2012, greater than 99% of all the ethylbenzene produced is used for this purpose.
Niche uses
Ethylbenzene is added to gasoline as an anti-knock agent, meaning it reduces engine knocking and increases the octane rating. Ethylbenzene is often found in other manufactured products, including pesticides, cellulose acetate, synthetic rubber, paints, and inks. Used in the recovery of natural gas, ethylbenzene may be injected into the ground.
Production
Ethylbenzene is produced in on a large scale by combining benzene and ethylene in an acid-catalyzed chemical reaction. Approximately 24,700,000 tons were produced in 1999.
Industrial Methods
Ethylbenzene is produced in on a large scale by combining benzene and ethylene in an acid-catalyzed chemical reaction:
- C
6H
6 + C
2H
4 → C
6H
5CH
2CH
3
In 2012, more than 99% of ethylbenzene was produced in this way. Thus, manufacturers of ethylbenzene are the major buyers of benzene, claiming more than half of total output.
Small amounts of ethylbenzene are recovered from the mix of xylenes by superfractioning, an extension of the distillation process.
In the 1980s a zeolite-based process using vapor phase alkylation offered a higher purity and yield. Then a liquid phase process was introduced using zeolite catalysts. This offers low benzene-to-ethylene ratios, reducing the size of the required equipment and lowering byproduct production.