You have no items in your shopping cart
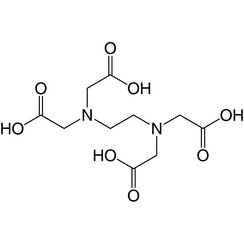
Ethylenediamine tetraacetic acid
Ethylenediaminetetraacetic acid (EDTA), also known by several other names, is a chemical used for both industrial and medical purposes. It was synthesised for the first time in 1935 by Ferdinand Münz.
It is an aminopolycarboxylic acid and a colourless, water-soluble solid. Its conjugate base is ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ("six-toothed") ligand and chelating agent, i.e., its ability to sequester metal ions such as Ca2+ and Fe3+. After being bound by EDTA into a metal complex, metal ions remain in solution but exhibit diminished reactivity. EDTA is produced as several salts, notably disodium EDTA, calcium disodium EDTA, and tetrasodium EDTA (typically as the hydrate)