You have no items in your shopping cart
Phosphoric acid
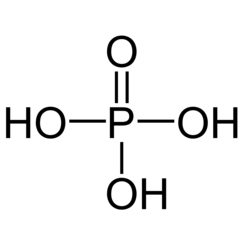
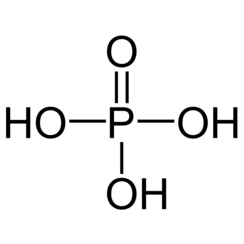
Phosphoric acid, also known as orthophosphoric acid or phosphoric(V) acid, is a weak acid with the chemical formula H
3PO
4. It is normally encountered as a colorless syrup of 85% concentration in water. The pure compound is a colorless solid.
All three hydrogens are acidic to varying degrees and can be lost from the molecule as H+ ions (protons). When all three H+ ions are removed, the result is an orthophosphate ion PO43−, commonly called "phosphate". Removal of one or two protons gives dihydrogen phosphate ion H
2PO−
4, and the hydrogen phosphate ion HPO2−
4, respectively. Orthophosphoric acid also forms esters, called organophosphates.
Phosphoric acid is commonly encountered in chemical laboratories as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. Although phosphoric acid does not meet the strict definition of a strong acid, the 85% solution can still severely irritate the skin and damage the eyes.
The name "orthophosphoric acid" can be used to distinguish this specific acid from other "phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclatur
Food-grade phosphoric acid (additive E338) is used to acidify foods and beverages such as various colas and jams, providing a tangy or sour taste. Soft drinks containing phosphoric acid, which would include Coca-Cola, are sometimes called phosphate sodas or phosphates. Phosphoric acid in soft drinks has the potential to cause dental erosion. Phosphoric acid also has the potential to contribute to the formation of kidney stones, especially in those who have had kidney stones previously.
Specific applications of phosphoric acid include:
- In anti-rust treatment by phosphate conversion coating or passivation
- As an external standard for phosphorus-31 nuclear magnetic resonance.
- In phosphoric acid fuel cells.
- In activated carbon production.
- In compound semiconductor processing, to etch Indium gallium arsenide selectively with respect to indium phosphide.
- In microfabrication to etch silicon nitride selectively with respect to silicon dioxide.
- As a pH adjuster in cosmetics and skin-care products.
- As a sanitizing agent in the dairy, food, and brewing industries.