You have no items in your shopping cart
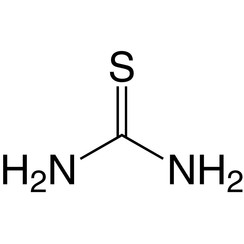
Thiourea
Thiourea is an organosulfur compound with the formula SC(NH2)2. It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refers to a broad class of compounds with the general structure (R1R2N)(R3R4N)C=S. Thioureas are related to thioamides, e.g. RC(S)NR2, where R is methyl, ethyl, etc
Applications
Thiourea per se has few applications. It is mainly consumed as a precursor to thiourea dioxide, which is a common reducing agent in textile processing.[6]
Other uses
Other industrial uses of thiourea include production of flame retardant resins, and vulcanization accelerators.
Thiourea is used as an auxiliary agent in diazo paper, light-sensitive photocopy paper and almost all other types of copy paper.
It is also used to tone silver-gelatin photographic prints.
Thiourea is used in the Clifton-Phillips and Beaver bright and semi-bright electroplating processes. It is also used in a solution with tin(II) chloride as an electroless tin plating solution for copper printed circuit boards.