You have no items in your shopping cart
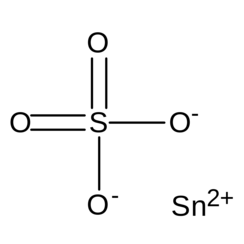
Tin(II) sulphate
€44,63
Excl. tax
Tin(II) sulfate (SnSO4) is a chemical compound. It is a white solid that can absorb enough moisture from the air to become fully dissolved, forming an aqueous solution; this property is known as deliquescence. It can be prepared by a displacement reaction between metallic tin and copper(II) sulfate:
- Sn (s) + CuSO4 (aq) → Cu (s) + SnSO4 (aq)
Tin(II) sulfate is a convenient source of tin(II) ions uncontaminated by tin(IV) species.