You have no items in your shopping cart
Zinc acetate
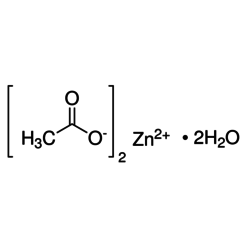
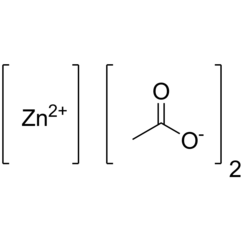
Zinc acetate is a salt with the formula Zn(CH3CO2)2, which commonly occurs as the dihydrate Zn(CH3CO2)2·2H2O. Both the hydrate and the anhydrous forms are colorless solids that are commonly used in chemical synthesis and as dietary supplements. Zinc acetates are prepared by the action of acetic acid on zinc carbonate or zinc metal. When used as a food additive, it has the E number E650.
Uses
Dietary and medicinal applications
Zinc acetate has been used in lozenges for treating the common cold. Zinc acetate can also be used to treat zinc deficiencies. As an oral daily supplement it is used to inhibit the body's absorption of copper as part of the treatment for Wilson's disease. Zinc acetate is also sold as an astringent in the form of an ointment, a topical lotion, or combined with an antibiotic such as erythromycin for the topical treatment of acne. It is commonly sold as a topical anti-itch ointment.
Industrial applications
Industrial applications include wood preservation, manufacturing other zinc salts, polymers, manufacture of ethyl acetate, as a dye mordant, and analytical reagent. It is used in commercial nuclear power plants as a plating inhibitor on primary water piping.