You have no items in your shopping cart
Copper(II) chloride dihydrate ≥98.5%, extra pure
- Buy 2 and save 5%
- Buy 10 and save 10%
What is copper(II) chloride?
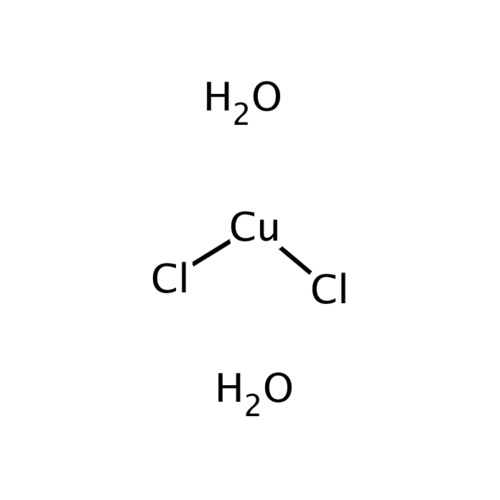
Copper(II) chloride is a chemical compound and a copper salt of hydrochloric acid with the formula CuCl2. Anhydrous copper(II) chloride is a brown powder that is very hygroscopic. The incorporation of water molecules into the crystal structure produces copper(II) chloride dihydrate, a blue-green solid with the formula CuCl2 · 2 H2O. The copper chloride, which is free of water of crystallization, melts in moist air to a brown paste, while the dihydrate is stable in air. The water of crystallization is driven out of the dihydrate by heating it to over 100 °C and the brown, anhydrous form remains.
There is also basic copper(II) chloride (copper(II) oxychloride, Cu2Cl(OH)3), which is insoluble in water and is referred to as the tetrahydrate as Braunschweig green.
All compounds exhibit a blue-green flame color in a burner flame, caused by the Cu2+ ions.
What is copper(II) chloride used for?
Cupric chloride is used as a catalyst in organic syntheses, e.g. used in the production of the textile dye aniline black and oxychlorination. It is also used in pyrotechnics to produce green flames, in copper etching (in a mixture with hydrochloric acid), in photography for bleaching negatives, as basic copper chloride in fruit growing and viticulture against fungal diseases and for removing soot from oil stoves.
Buy copper chloride?
Buy the best quality copper chloride at Laboratoriumdiscounter.nl. Produced in Europe and for a friendly price! So buy your copper(II) chloride at Laboratoriumdiscounter.nl Delivered quickly and always with volume discount.
What Safety Precautions Should I Take Before Using Chemicals?
It is always wise to work as safely as possible when working with chemicals. Even substances that do not seem dangerous at first can cause enormous damage if they get into your eyes. Therefore, always wear safety goggles. You also want to prevent chemicals from ending up on your skin, which is why it is important to always use good gloves or disposable gloves.
Respiratory protection is necessary for volatile substances, vaporous liquids and solids that dust. There are many different types of filters, so you should always refer to the MSDS to find out which filter you need. Many filters also specify which substances they are for.
In some cases it may be necessary to protect the whole body, in which case a plastic overall is needed, you can find it here.
When working with flammable and oxidizing substances, it is important to always have fire extinguishers and absorbents at hand. It is also useful to be able to clean up spills of aggressive chemicals immediately with absorbents and to dispose of them in accordance with international and/or local legislation.
If you need more information on how to handle a specific substance, always consult the safety data sheet. You can find this on the product page or request it via [email protected]
Technical data
Empirical formula CuCl2 2 H2O
Molar mass (M) 170.48 g/mol
Density (D) 2.54
Solubility 1150 g/l (H2O, 20 °C)
ADR 8 III • WGK 3
CAS No.[10125-13-0]
EC-No. 231-210-2 • UN 2802
Downloads
$$$$$
Hazard statements
H290 May be corrosive to metals.
H302 + H312 Harmful if swallowed and in contact with skin.
H315 Causes skin irritation.
H318 Causes serious eye damage.
H410 Very toxic to aquatic life with long lasting effects.
Precautions - prevention
P273 Avoid release to the environment.
P280 Wear protective gloves / eye protection.
Precautions - response
P301 + P330 + P331 IF SWALLOWED: rinse mouth. Do NOT induce vomiting.
P302 + P352 IF ON SKIN: Wash with plenty of soap and water.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P312 Call a POISON CENTER / doctor if you feel unwell.