You have no items in your shopping cart
Ethyl acetate 99.8+%
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Ethyl Acetate?
Ethyl acetate is a widely used solvent for chemical reactions and for extractions. It is present in nail polishes and nail polish remover and can be used for the removal of caffeine from coffee beans and tea leaves, although this is usually done with liquid CO2 (caffeine extraction with supercritical carbon dioxide).
Ethyl acetate is also present in candies, perfumes, and fruits because it has a fruity odor like many other esters.
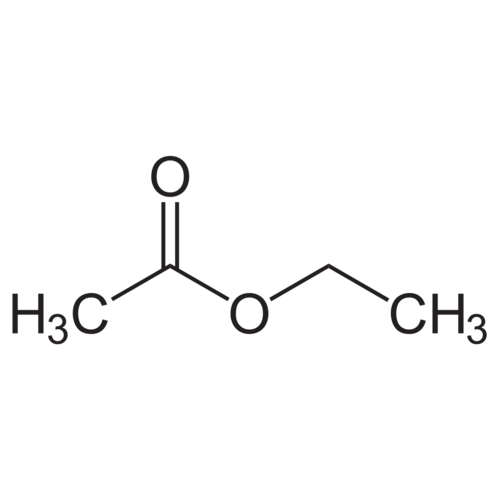
Ethyl acetate is the ester of ethanol and acetic acid. The systematic IUPAC name is ethyl ethanoate. This colorless liquid has a characteristic sweet smell, known from certain glues and nail polish remover. Ethyl acetate, sometimes abbreviated as EtOAc or AcOEt, is widely produced.
Ethyl acetate has the chemical formula CH₃COOC₂H₅
What is ethyl acetate used for?
Ethyl acetate is a versatile solvent. Ethyl acetate is used as an extraction agent for decaffeinating coffee beans or as a natural flavoring agent for fruit and cognac notes, for flavoring lemonades, candies and medicines. It is naturally present in small amounts in rum and some other spirits. It is also found in wine in small amounts, especially when the grapes have been damaged by hail or rot; it then causes a solvent note in the wine.
Due to its strong solvent power, ethyl acetate is also used as part of nail polish removers and thinners, where it has largely replaced acetone as a solvent. It is one of the most commonly used solvents in adhesives.
In energy storage technology, ethyl acetate is used as an electrolyte. The advantage of organic liquids over aqueous electrolytes is that they function well at low temperatures. The disadvantage is the much lower energy density.
In entomology, it is the most commonly used means of killing insects.
Buy ethyl acetate?
You can buy ethyl acetate of the highest quality at Laboratoriumdiscounter. Not only available in different packagings, but also always a volume discount when purchasing multiple packagings!
Technical data
Formula: CH₃COOC₂H₅
MW: 88.11 g / mol
Boiling Pt: 77.1 ° C (1013 hPa)
Melting point: -83 ° C
Density: 0.902 g / cm³ (20 ° C)
Flash point: -4 ° C
CAS number: 141-78-6
UN: 1173
ADR: 3, II
$$$$$
Hazard statement code (H code)
H225 H319 H336
Highly flammable liquid and vapor. Causes serious eye irritation.
May cause drowsiness or dizziness.
Preventive code (P code)
P210 P261 P305 + P351 + P338
Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. Do not smoke.
Avoid inhalation of vapor.
IF IN EYES: Rinse cautiously with water for several minutes. remove contact lenses, if possible; keep rinsing.
Additional hazard information (EU)
EUH066 Repeated exposure may cause skin dryness or cracking.


%%%%%
| MSDS Ethylacetaat (NL) |
| MSDS Ethylacetat (DE) |
| MSDS Ethyl acetate (EN) |
| MSDS Acétate d'éthyle (FR) |
| MSDS Acetato de etilo (ES) |