You have no items in your shopping cart
Acetonitrile
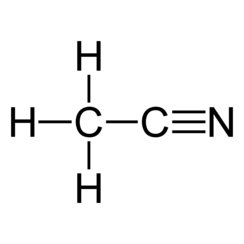
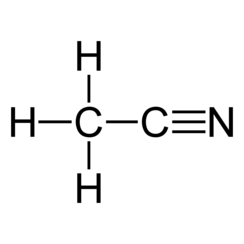
Acetonitrile is the chemical compound with the formula CH
3CN. This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene.
In the laboratory, it is used as a medium-polarity solvent that is miscible with water and a range of organic solvents, but not saturated hydrocarbons. It has a convenient liquid range and a high dielectric constant of 38.8. With a dipole moment of 3.92 D, acetonitrile dissolves a wide range of ionic and nonpolar compounds and is useful as a mobile phase in HPLC and LC–MS. The N≡C−C skeleton is linear with a short C≡N distance of 1.16 Å.
Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas
Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separating tower to separate the butadiene.
It is widely used in battery applications because of its relatively high dielectric constant and ability to dissolve electrolytes. For similar reasons it is a popular solvent in cyclic voltammetry.
Its ultraviolet transparency UV cutoff, low viscosity and low chemical reactivity make it a popular choice for high-performance liquid chromatography (HPLC).
Acetonitrile plays a significant role as the dominant solvent used in the manufacture of DNA oligonucleotides from monomers.
Industrially, it is used as a solvent for the manufacture of pharmaceuticals and photographic film.
Organic synthesis
Acetonitrile is a common two-carbon building block in organic synthesis of many useful chemicals, including acetamidine hydrochloride, thiamine, and α-napthaleneacetic acid. Its reaction with cyanogen chloride affords malononitrile.
Ligand in coordination chemistry
Acetonitrile is a ligand in many transition metal nitrile complexes. Being weakly basic, it is an easily displaceable ligand. For example, bis(acetonitrile)palladium dichloride is prepared by heating a suspension of palladium chloride in acetonitrile:
- PdCl
2 + 2 CH
3CN → PdCl
2(CH
3CN)
2
A related complex is [Cu(CH3CN)4]+. The CH
3CN groups in these complexes are rapidly displaced by many other ligands.