You have no items in your shopping cart
Ammonium cerium(IV) nitrate
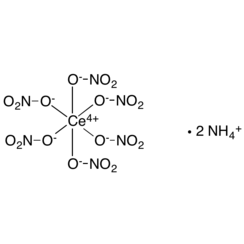
Ceric ammonium nitrate (CAN) is the inorganic compound with the formula (NH4)2Ce(NO3)6. This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard oxidant in quantitative analysis.
Applications in organic chemistry
In organic synthesis, CAN is useful as an oxidant for many functional groups (alcohols, phenols, and ethers) as well as C–H bonds, especially those that are benzylic. Alkenes undergo dinitroxylation, although the outcome is solvent-dependent. Quinones are produced from catechols and hydroquinones and even nitroalkanes are oxidized.
CAN provides an alternative to the Nef reaction; for example, for ketomacrolide synthesis where complicating side reactions usually encountered using other reagents. Oxidative halogenation can be promoted by CAN as an in situ oxidant for benzylic bromination, and the iodination of ketones and uracil derivatives.
For the synthesis of heterocycles
Catalytic amounts of aqueous CAN allow the efficient synthesis of quinoxaline derivatives. Quinoxalines are known for their applications as dyes, organic semiconductors, and DNA cleaving agents. These derivatives are also components in antibiotics such as echinomycin and actinomycin. The CAN-catalyzed three-component reaction between anilines and alkyl vinyl ethers provides an efficient entry into 2-methyl-1,2,3,4-tetrahydroquinolines and the corresponding quinolines obtained by their aromatization.
As a deprotection reagent
CAN is traditionally used to release organic ligands from metal carbonyls. In the process, the metal is oxidised, CO is evolved, and the organic ligand is released for further manipulation.[3] For example, with the Wulff–Dötz reaction an alkyne, carbon monoxide, and a chromium carbene are combined to form a chromium half-sandwich complex and the phenol ligand can be isolated by mild CAN oxidation.