You have no items in your shopping cart
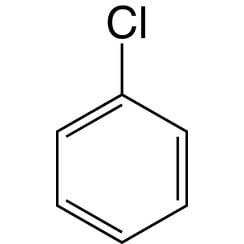
Chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
The major use of chlorobenzene is as an intermediate in the production of commodities such as herbicides, dyestuffs, and rubber. Chlorobenzene is also used as a high-boiling solvent in many industrial applications as well as in the laboratory. Chlorobenzene is nitrated on a large scale to give a mixture of 2-nitrochlorobenzene and 4-nitrochlorobenzene, which are separated. These mononitrochlorobenzenes are converted to related 2-nitrophenol, 2-nitroanisole, bis(2-nitrophenyl)disulfide, and 2-nitroaniline by nucleophilic displacement of the chloride, with respectively sodium hydroxide, sodium methoxide, sodium disulfide, and ammonia. The conversions of the 4-nitro derivative are similar.
Chlorobenzene once was used in the manufacture of certain pesticides, most notably DDT, by reaction with chloral (trichloroacetaldehyde), but this application has declined with the diminished use of DDT. At one time, chlorobenzene was the main precursor for the manufacture of phenol.
The reaction also has a byproduct of salt. The reaction is known as the Dow process, with the reaction carried out at 350 °C using fused sodium hydroxide without solvent. Labeling experiments show that the reaction proceeds via elimination/addition, through benzyne as the intermediate