You have no items in your shopping cart
Dichloromethane
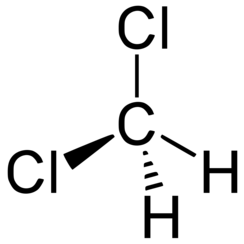
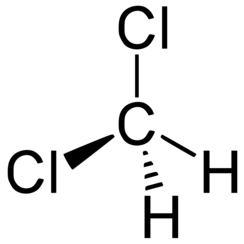
Dichloromethane (DCM or methylene chloride) is an organochloride compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is polar, and miscible with many organic solvents.
DCM's volatility and ability to dissolve a wide range of organic compounds makes it a useful solvent for many chemical processes. In the food industry, it has been used to decaffeinate coffeeand tea as well as to prepare extracts of hops and other flavorings. Its volatility has led to its use as an aerosol spray propellant and as a blowing agent for polyurethane foams.
Specialized uses
The chemical compound's low boiling point allows the chemical to function in a heat engine that can extract mechanical energy from small temperature differences. An example of a DCM heat engine is the drinking bird. The toy works at room temperature. It is also used as the fluid in Christmas lights that have the colored bubbling tube above a lamp as a source of heat and a small amount of rock salt to provide thermal mass and a nucleation site for the phase changing solvent.
DCM chemically welds certain plastics. For example, it is used to seal the casing of electric meters. Often sold as a main component of plastic welding adhesives, it is also used extensively by model building hobbyists for joining plastic components together. It is commonly referred to as "Di-clo."
It is used in the garment printing industry for removal of heat-sealed garment transfers, and its volatility is exploited in novelty items: bubble lightsand jukebox displays.
DCM is used in the material testing field of civil engineering; specifically it is used during the testing of bituminous materials as a solvent to separate the binder from the aggregate of an asphalt or macadam to allow the testing of the materials.
Dichloromethane extract of Asparagopsis taxiformis, a seaweed fodder for cattle, has been found to reduce their methane emissions by 79%.