You have no items in your shopping cart
L(+)-Ascorbic acid ≥99 %, BP/USP/EP/FCC/E300, Foodgrade
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Ascorbic Acid?
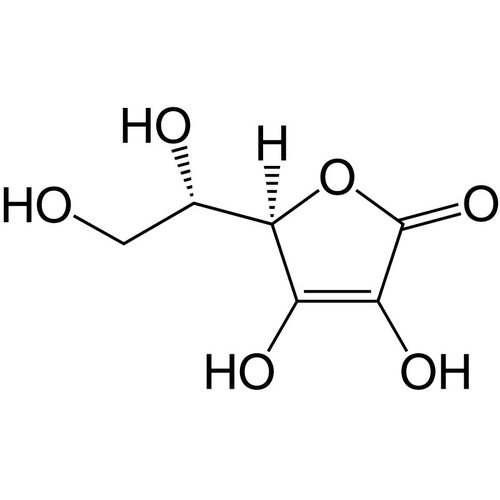
Ascorbic acid is a colorless, odorless, crystalline, water-soluble solid with a sour taste. It is an organic acid, more precisely a vinylogic carboxylic acid; their salts are called ascorbates. Ascorbic acid exists in four different stereoisomeric forms, but only L-(+)-ascorbic acid has biological activity. An important property in humans and some other species is its physiological effect as a vitamin. Deficiency can manifest in humans as scurvy. The name is therefore derived from the Latin name of the disease, scorbutus, with the negative prefix a- (weg-, un-), that is, the 'anti-scorbutic acid'. Since ascorbic acid is easily oxidizable, it acts as a reductone and is used as an antioxidant.
L-(+)-ascorbic acid and its derivatives (derivatives) with the same effect are summarized under the name vitamin C. The collective term vitamin C therefore also includes substances that can be converted into L-(+)-ascorbic acid in the body, such as dehydroascorbic acid (DHA).
In food, vitamin C is mainly found in fruits and vegetables. Citrus fruits such as oranges, lemons and grapefruits - if they are ripe immediately after harvest - contain a lot of vitamin C. Kale has the highest vitamin C content of all cabbages (105–120 mg / 100 g of edible matter). Red cabbage, white cabbage and sauerkraut are also sources of vitamin C. Sauerkraut has long been important in the maritime industry, where a long shelf life food rich in vitamin C was needed. The highest natural vitamin C concentrations were found in the bush plum and camu camu. In sauerkraut and cabbage vegetables, ascorbic acid is bound in the form of ascorbents A and B (C-2-scatyl-L-ascorbic acid). When the vegetables are cooked, the molecules break down into L-ascorbic acid and 3-hydroxyindole, so they can contain more vitamin C when cooked than when raw. If the food is cooked for too long, the vitamin will increasingly end up in the cooking water. Many types of vegetables contain ascorbic acid oxidase, which comes into contact with the vitamin and oxidizes it, especially during chopping. For example, with raw food that is not consumed immediately, this leads to significant vitamin C losses.
The following information is only intended as a guideline, the actual values are highly dependent on the type of plant, the nature of the soil, the climate during growth, the storage period after harvest, the storage conditions and the preparation. For example, the wheat grain does not contain vitamin C, which is only created during germination.
What is Ascorbic Acid used for?
Ascorbic acid is mainly used as an antioxidant. It is added to many foods as a preservative or as a redning aid, for example in the production of cooked sausages, under number E 300. Other E numbers of ascorbic acid derivatives are E 301 (sodium ascorbate), E 302 (calcium ascorbate), E 304a (ascorbyl palmitate) and E 304b (ascorbyl stearate). Natural cloudy apple juice can be mixed with ascorbic acid during production and becomes significantly lighter because the quinones present in the natural juice, which are formed during the pressing process by the oxidation of phenols with atmospheric oxygen and the enzyme polyphenol oxidase, cause a brown color. are reduced again. Ascorbyl palmitate is used to prevent the auto-oxidation of fats and thus prevent them from becoming rancid. The addition of ascorbic acid to flour as a flour treatment agent is intended to increase the gas holding capacity and volume of the dough. This can be explained by the formation of additional disulphide bridges between the adhesive strands of the dough. In the pharmaceutical sector, ascorbic acid is also used as an antioxidant to stabilize pharmaceutical products.
In the kitchen, ascorbic acid (usually called "vitamin C powder" in recipes) is used so that cut fruits (usually apples and bananas) stay fresh longer and do not turn brown.
Because of its reducing properties, ascorbic acid is occasionally used as a developing agent in photographic developers.
Why buy Ascorbic acid at Laboratoriumdiscounter.nl
If you need ascorbic acid of the highest quality, you have come to the right place at Laboratoriumdiscounter! Not only is this Ascorbic Acid of the highest quality, it is also of food grade and pharmaceutical quality. So you are guaranteed a good product. Delivered quickly and available in different packaging!
Technical data
Empirical formula C6H8O6
Molar mass (M) 176.12 g/mol
Density (D) 1.65
Melting point (mp)190-192 °C
Solubility 333 g/l (H2O, 20 °C)
WCK 1
CAS No.[50-81-7]
EC-No. 200-066-2
Downloads