You have no items in your shopping cart
Sodium Thiosulfate Pentahydrate 99 +%, pure
- Buy 2 and save 5%
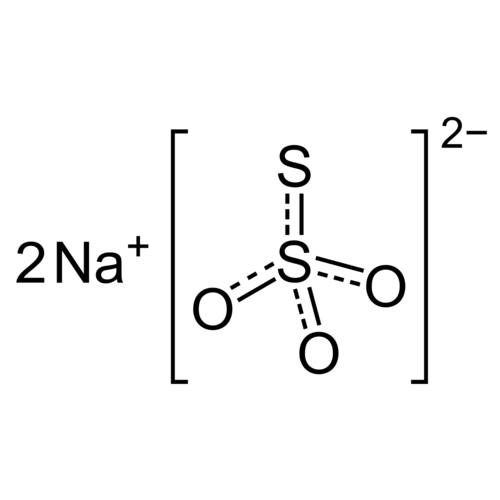
Sodium thiosulfate is the stable sodium salt of thiosulfuric acid.
use
Sodium thiosulfate can be used as a fixing salt in analog photography, in mining for the extraction of silver chloride from silver ores, and in electroplating for the production of gold and silver baths. In medicine, it is used as an antidote to cyanide poisoning, while forming less dangerous thiocyanate.
Sodium thiosulfate is used as an antichlor to end chlorine bleaching or disinfection processes. The chlorine is reduced to chloride (or hydrochloric acid) and hydrogen sulfate is formed.
In chemistry it is used to determine the iodine number, in iodometry thiosulfate is oxidized to tetrathionate.
It is also used as a pentahydrate in so-called heating pads. Bending a metal plate creates a crystallization core that initiates the exothermic crystallization. The pillow is regenerated by heating it in boiling water, causing the crystals to melt (loosen) again.
Technical data:
Empirical formula Na2S2O3 · 5 H2O
Molar mass (M) 248.18 g / mol
Density (D) 1.74
Melting point (mp) approx. 48 ° C
Solubility 701 g / l (H2O, 20 ° C)
WGK 1
CAS No. [10102-17-7]
EC no. 231-867-5