You have no items in your shopping cart
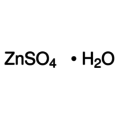
Zinc Sulphate Monohydrate ≥97%, pure
- Buy 2 and save 5%
Zinc sulphate (ZnSO4) is the zinc salt of sulfuric acid (common names: sulfuric acid zinc oxide, zinc vitriol, white vitriol, Vitriolum album, (white) copper smoke, (white) galitzenstein, eye stone). It forms a colorless crystal, in anhydrous form it is a white powder.
Zinc sulphate is used in the case of the paints, before the impregnation of wood and for the production of electrolytic zinc. Because the bactericidal effect of Zn2 + ions is greatly reduced, solutions of ZnSO4 (0.1–0.5%) are used as eye wash in conjunctivitis.
Zinc sulfate is also used:
in calico print
for the preservation of wood and hides
as a contractor
as an additive to varnish to make the oil medicated quickly
in fire silver
as a bactericidal agent in toothpaste
as a flotation aid in mining
It is also used as an emetic agent, colorant in the textile industry, trace nutrients in fertilizers and in precipitation baths in rayon extraction. Zinc sulphate is also used for the production of zinc sulphide pigments and other zinc compounds, for floating ores, for weighting cotton and in galvanic zinc baths.
Technical information
Empirical formula ZnSO4 H2O
Molar mass (M) 179.49 g / mol
Density (D) 3.2
Melting point (mp) 740 ° C
Solubility 350 g / l (H2O, 20 ° C)
ADR 9 III • WGK 3
CAS No. [7446-19-7]
EC no. 231-793-3 • UN no. 3077
Downloads
$$$$$
Hazard statements
H302 Harmful if swallowed
H318 Causes serious eye damage
H410 Very toxic to aquatic life with long lasting effects
Precautions - prevention
P273 Avoid release to the environment.
P280 Wear protective gloves / eye protection.
Precautions - response
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P310 Immediately call a POISON CENTER / doctor / ....