You have no items in your shopping cart
Strontium Nitrate 99.8 +% pure
- Buy 2 and save 5%
- Buy 6 and save 10%
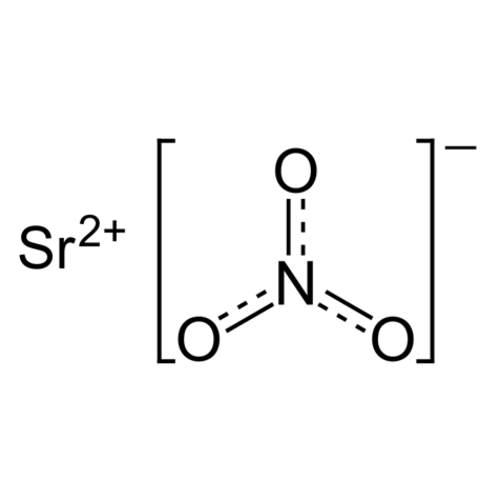
Strontium nitrate, Sr (NO3) 2, is the strontium salt of nitric acid. It is used as an oxidizing agent in pyrotechnics and gives it the deep red flame color typical of strontium.
The anhydrous strontium nitrate crystallizes above 31.3 ° C, the tetrahydrate below this temperature from an aqueous solution. Anhydrous strontium nitrate crystallizes in the cubic crystal system in the space group Pa3 (space group No. 205) with the lattice parameter a = 778.13 pm. There are four formula units in the unit cell. The crystals are isotypical for barium nitrate. The tetrahydrate forms monoclinic crystals with the space group C2 / c (No. 15), the lattice parameters a = 1112 pm, b = 1417 pm, c = 634 pm, β = 123.75 ° and four formula units in the unit cell.
-Use
Among other things, strontium nitrate is used. used in pyrotechnics. Together with magnesium powder, at high temperature, favored by z. B. 5-Amino-1H-tetrazole or hexamethylenetetramine, strontium (I) hydroxide (SrOH) is briefly generated. This is a strong emitter in the red spectral range and acts as the sole color emitter in bright and deeply saturated red pyrotechnic light packages.
-Technical information
Empirical formula Sr (NO3) 2
Molar mass (M) 211.63 g / mol
Density (D) 2.99
Boiling point (bp) 645 ° C
Melting point (mp) 570 ° C
Solubility 660 g / l (H2O, 20 ° C)
ADR 5.1 III • WGK 1
CAS No. [10042-76-9]
EC no. 233-131-9 • UN-no. 1507
Downloads
$$$$$
Hazard statements
H271 May cause fire or explosion; strong oxidiser
H318 Causes serious eye damage
Precautions - prevention
P210 Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. Do not smoke.
P280 Wear protective gloves / eye protection.
Precautions - response
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P310 Immediately call a POISON CENTER / doctor.