You have no items in your shopping cart
Nickel (II) sulfate 98 +% pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Nickel Sulfate?
Nickel(II) sulfate is the nickel salt of sulfuric acid. It consists of a nickel cation (Ni2+) and a sulfate anion (SO42−). When heated above 840 ° C, decomposition occurs producing toxic fumes (nickel monoxide, sulfur trioxide). The nickel(II) sulfate reacts slightly acidic in aqueous solution.
What is Nickel Sulphate used for?
Nickel(II) sulfate is the technically most important nickel compound. It is used to produce other nickel compounds and catalysts. The aqueous solutions of nickel(II) sulfate and nickel(II) chloride NiCl2 are used for the electrodeposition of metallic nickel layers. It is also used in dyeing as a mordant and in the manufacture of gas masks.
Technical information
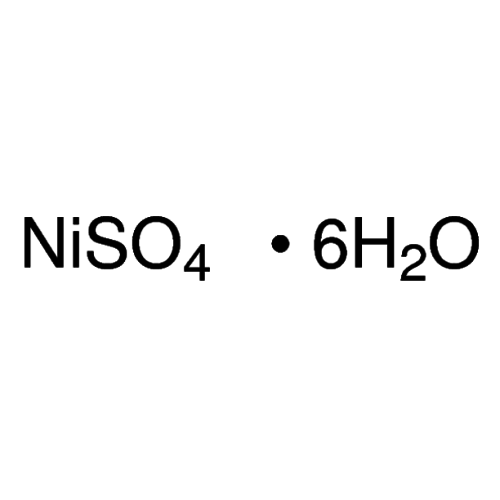
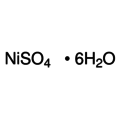
Empirical formula NiSO4 6 H2O
Molar mass (M) 262.86 g / mol
Density 2.07
Melting point (mp)> 100 ° C (dec.)
Solubility: 650 g / l (H2O, 20 ° C)
ADR 9 III • WGK 3
CAS No. [10101-97-0]
EC no. 232-104-9 • UN No. 3077
Downloads
$$$$$
Hazard statements
H302 + H332 Harmful if swallowed and if inhaled.
H315 Causes skin irritation.
H317 May cause an allergic skin reaction.
H334 May cause allergy or asthma symptoms or breathing difficulties if inhaled
cause.
H341 Suspected of causing genetic defects.
H350i May cause cancer by inhalation.
H360D May damage the unborn child.
H372 Causes damage to organs through prolonged or repeated exposure.
H410 Very toxic to aquatic life with long lasting effects.
Precautions - prevention
P260 Do not breathe dust.
P273 Avoid release to the environment.
P280 Wear protective gloves / eye protection.
Precautions - response
P302 + P352 IF ON SKIN: Wash with plenty of soap and water.
P304 + P340 IF INHALED: Remove to fresh air and keep at rest in a position suitable for use
makes breathing easier.
P342 + P311 In case of respiratory symptoms: Call a POISON CENTER / doctor.