You have no items in your shopping cart
Manganese(II) chloride tetrahydrate 98 +%, extra pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Manganese Chloride?
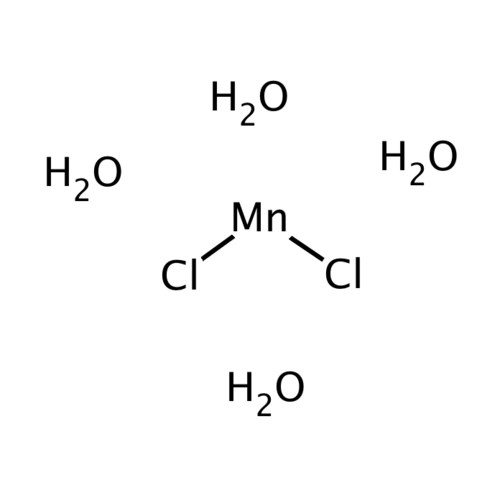
Manganese(II) chloride refers to a series of chemical compounds with the formula MnCl2(H2O)x, where the value of x can be 0, 2 or 4. The tetrahydrate with the formula MnCl2 4H2O is the most common form of "chloride" of manganese(II). There is also an anhydrous form and a dihydrate with the formula MnCl2 2H2O. Like many Mn(II) compounds, these salts pink, with the pallor of the color characteristic of transition metal complexes with high-spin d51 configurations.It is a paramagnetic salt.
What is Manganese Chloride used for?
Manganese chloride is mainly used in the production of dry cell batteries. It is the precursor of the anti-knock compound (methylcyclopentadienyl) manganese tricarbonyl.
Buy manganese chloride?
The best quality manganese chloride can be found at Laboratoriumdiscounter. Available in different packaging and always with volume discount.
What Safety Precautions Should I Take Before Using Chemicals?
It is always wise to work as safely as possible when working with chemicals. Even substances that do not seem dangerous at first can cause enormous damage if they get into your eyes. Therefore, always wear safety goggles. You also want to prevent chemicals from ending up on your skin, which is why it is important to always use good gloves or disposable gloves.
Respiratory protection is necessary for volatile substances, vaporous liquids and solids that dust. There are many different types of filters, so you should always refer to the MSDS to find out which filter you need. Many filters also specify which substances they are for.
In some cases it may be necessary to protect the whole body, in which case a plastic overall is needed, you can find it here.
When working with flammable and oxidizing substances, it is important to always have fire extinguishers and absorbents at hand. It is also useful to be able to clean up spills of aggressive chemicals immediately with absorbents and to dispose of them in accordance with international and/or local legislation.
If you need more information on how to handle a specific substance, always consult the safety data sheet. You can find this on the product page or request it via [email protected]
Technical information
Empirical formula MnCl2 · 4 H2O
Molar mass (M) 197,91 g/mol
Dichtheid 2,01
Melting point (mp)58 °C
Oplosbaarheid: 1980 g/l (H2O, 20 °C)
ADR 6.1 III • WGK 2
CAS No.[13446-34-9]
EG-Nr. 231-869-6 • UN-Nr. 3288
Downloads
$$$$$
Gevarenaanduidingen
H301 Giftig bij inslikken
H318 Veroorzaakt ernstig oogletsel
H373 Kan schade aan organen (hersenen) veroorzaken bij langdurige of herhaalde
blootstelling (na inademing)
Voorzorgsmaatregelen - preventie
P260 Stof / rook / gas / nevel / damp / spuitnevel niet inademen.
P280 Beschermende handschoenen / oogbescherming / gelaatsbescherming dragen.
Voorzorgsmaatregelen - reactie
P301 + P310 NA INSLIKKEN: onmiddellijk een ANTIGIFCENTRUM / arts raadplegen.
P305 + P351 + P338 BIJ CONTACT MET DE OGEN: voorzichtig afspoelen met water gedurende een
aantal minuten; contactlenzen verwijderen, Indian mogelijk; blijven spoelen.
P308 + P313 NA (mogelijke) blootstelling: een arts raadplegen.