You have no items in your shopping cart
Lithium Carbonate 99+% Extra Pure
- Buy 2 and save 5%
What is Lithium Carbonate?
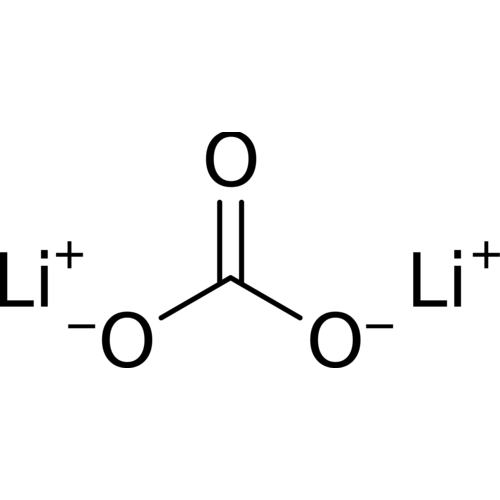
Lithium carbonate is a chemical compound with the formula Li2CO3. It is a colorless salt with a density of 2.11, a molecular weight of 73.89 g/mol, widely used in the treatment of metal oxides.
Lithium carbonate is an important industrial compound in metallurgy, glassware and ceramics, in electrochemistry.
What is Lithium Carbonate used for?
In particular, it is used with silica to form soldering fluxes. Glasses produced with lithium carbonate are used to make ovens. Specific glasses and ceramic glazes are produced with lithium carbonate, rather than sodium or potassium carbonate.
Added to aluminum trifluoride AlF3, the lithium fluoride forms LiF which improves the properties of the electrolyte from which the aluminum is extracted. It is involved in the manufacture of most lithium-ion battery cathodes, which are made from cobalt dioxide and lithium LiCoO2.
Lithium carbonate can also be used as a carbon dioxide detector.
Buy lithium carbonate?
You can buy lithium carbonate of the highest quality at Laboratoriumdiscounter.nl. Not only competitively priced but also delivered quickly. Always with volume discount and available in different packaging.
Technical data:
Empirical formula Li2CO3
Molar mass (M) 73.89 g/mol
Density 2.11
Boiling point (bp) 1300 °C
Melting point (mp)720 °C
Solubility: 13 g/l (H2O, 20 °C)
WCS 1
CAS No.[554-13-2]
EC-No. 209-062-5
Downloads
$$$$$
Hazard statements
H302 Harmful if swallowed.
H319 Causes serious eye irritation.
Precautions - prevention
P280 Wear protective gloves / eye protection.
Precautions - response
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P337 + P313 If eye irritation persists: Get medical advice / attention.