You have no items in your shopping cart
Sodium Metasilicate Pentahydrate 98.5+% Pure
- Buy 2 and save 5%
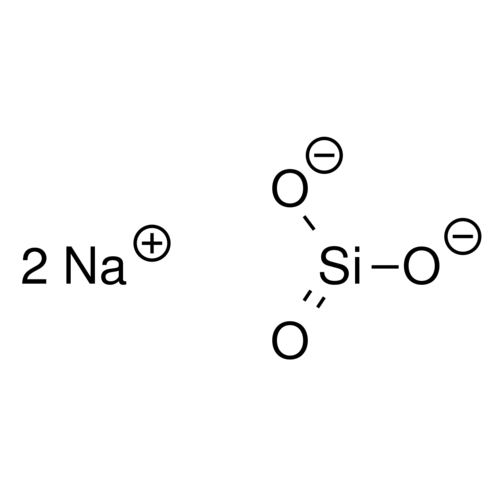
Sodium (meta)silicate (or disodium salt of silicic acid or disodium trixosilicate) is a chemical with the formula Na2SiO3, odorless and highly soluble in water. It is a strong base and forms very alkaline solutions, corrosive to the skin and mucous membranes (pH 13 in 1% solution).
Sodium (meta)silicate is naturally formed by reacting silicon dioxide with molten sodium carbonate. Sodium silicate and carbon dioxide are obtained.
Na2CO3 + SiO2 → Na2SiO3 + CO2
anhydrous form (it then appears as a translucent to white crystalline solid with the formula Na2SiO3);
hydrated form (Na2SiO3.nH2O), it is sometimes called "liquid glass".
It is one of a family of related compounds, including sodium orthosilicate, Na4SiO4, sodium pyrosilicate Na6Si2O7, and other related compounds, all of which are glassy, highly alkaline, colorless, and soluble in water.
Sodium silicate is stable in a neutral or alkaline pH solution.
In an acidic solution, silicate ion reacts with hydrogen ions to form silicic acid, which when heated and roasted forms silica gel, a hard, glass-like substance.
-Applications
in cement;
as a flame retardant;
in certain refractory materials;
in the textile industry;
in certain cleaning products (domestic or industrial, such as detergents, disinfectants, food cleaning products);
in the wood processing industry;
in the metallurgical industry (stripper, degreaser);
in some glues, paints and bleaches;
for the preparation of cosmetic products;
in the automotive industry;
in cardboard, to glue the flute (or microflute) to one or more flat plates, or several grooves between them, or several sheets of corrugated cardboard between them. When it goes through high-temperature machines, the sodium silicate polymerizes and bonds the sheets of paper tightly together;
in foodstuffs, as a food additive, then regulated under number E550, as an anti-caking agent and – with great success in the early 20th century – for the preservation of eggs. When fresh eggs are submerged in a sodium silicate solution, bacteria that can break down the egg are kept out and the humidity is maintained, allowing the egg to be stored for up to nine months. It was necessary to pierce the shell of the eggs thus preserved before being cooked in boiling water to allow the steam to escape as the shell was no longer porous (This variant is not food grade so cannot be used as an additive);
as a quick repair putty: sodium silicate and magnesium silicate dissolved in water are used in thick paste to repair exhaust pipes. When the exhaust system reaches its operating temperature, the heat dries the paste that polymerizes into a kind of glass, allowing repair (temporarily, because relatively fragile anyway);
as a repair kit in liquid and hot environments (it was used in 2011 to seal cracks after the accident at the Fukushima nuclear power plant).
Sodium silicate in solution can seal leaks in connections (e.g. cylinder head gasket, with "liquid glass" injected through the radiator of a car for example and sent to the hot parts of the engine, because at 100-105°C sodium silicate loses its water molecules to form a to form a very strong seal that only melts above 810°C A head gasket repaired with sodium silicate can remain sealed for up to two years or more, very quickly sealed (within minutes) This type of use is only possible in an environment that exceeds the polymerization temperature of 100-105°C reached;
as a corrosion inhibitor in certain water circuits;
as a growth substrate for certain algae in aquaculture farms;
for the (former) manufacture of ammunition, e.g. for the black powder revolver cartridges produced by the Colt's Manufacturing Company from 1851 to 1873, particularly during the American Civil War. The sodium silicate sealed the combustible nitro papers together to form a tapered paper cartridge containing the black powder, and bonded the lead bullet or tapered bullet to the open end of the paper "cartridge". These cartridges glued with sodium silicate were inserted in their entirety into the barrels of revolvers, which accelerated the reload time. This custom was dethroned by the invention of brass-jacketed cartridges of 187310,11;
in the drilling industry: mixed with the drilling fluid, it stabilizes the walls of boreholes and prevents the collapse or drilling of the walls, especially in clay layers or in formations containing swelling clay minerals such as smectite or montmorillonite;
for reinforcing masonry or concrete, stucco, plaster, etc. (i.e. made less porous or water-repellent by a chemical reaction with the excess Ca(OH)2 (portlandite) present in the concrete, which binds the silicates permanently, making their surface hardens and becomes water repellent.It is generally recommended to apply this treatment only after the first curing time (7 days or more, depending on the conditions);
to deactivate vehicle engines: To permanently take cars out of traffic, a cheap solution of sodium silicate is used to destroy the engine quickly and permanently. The mineral oil was replaced by two liters of solution, which precipitated and permanently damaged the gears and pistons within minutes12. For example, this procedure is mandatory in the United States under the Car Allowance Rebate System (CARS) program12,13;
to produce refractory materials, as a binder of non-combustible and insulating materials such as vermiculite and perlite.
-Technical data:
Formula: Na2SiO3 - 5H2O
Mol : 212.16 g/mol
CAS: 10213-79-3
UN 3253 III, 8
Downloads
$$$$$
signal word
• Hazard Statements
• Precautions
- Prevention
: Danger
: H290 - May be corrosive to metals. H314 - Causes severe burns
and eye injury. H335 - May cause respiratory irritation.
: P261 - Inhalation of dust/fume/gas/mist/vapours/spray