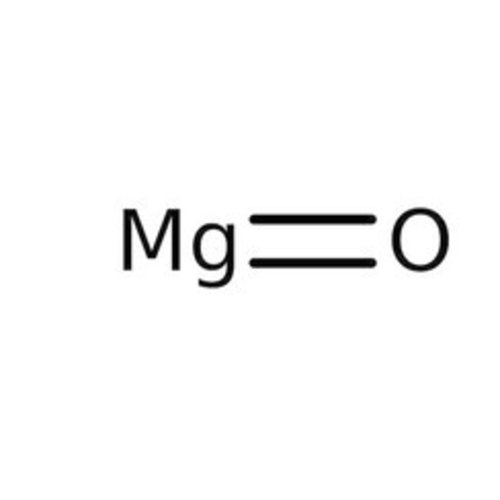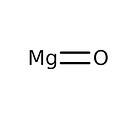You have no items in your shopping cart
Magnesium Oxide 98+% Ph. EUR. light
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Magnesium Oxide
Magnesium oxide, commonly referred to as magnesia, has the formula MgO and is in the form of a basic white powder that absorbs water and carbon dioxide in the atmosphere.
Most magnesium oxide is currently extracted from magnesium carbonate MgCO3, which forms certain minerals such as magnesite, or from magnesium chloride extracted underground from seawater or brine.
The first way uses a simple calcination: magnesium carbonate, heated from 700 to 1000 ° C, decomposes into magnesium oxide and carbon dioxide:
MgCO3 → MgO + CO2.
The second way involves two steps:
Magnesium hydroxide Mg(OH)2 is precipitated by adding lime to a brine concentrated in magnesium chloride:
MgCl2 + CaO + H2O → Mg(OH)2 + CaCl2;
the calcination of the magnesium hydroxide thus obtained yields the magnesium oxide:
Mg(OH)2 → MgO + H2O.
The properties of the magnesium oxide obtained are strongly dependent on the temperature at which the calcination is carried out:
between 700°C and 1000°C, a product is obtained that is used in many industrial applications, for example as a pigment for paints, filler for paper and certain synthetic materials, neutralizing agent;
between 1,000°C and 1,500°C, a chemically less reactive product is obtained that is very suitable for applications that require slow degradation: fertilizers, nutritional supplements for livestock;
between 1,500°C and 2,000°C, the magnesium oxide obtained is called "sintered" and is particularly stable even at very high temperatures. It is mainly used as a refractory material: bricks for the construction of furnaces, lining of crucibles used in metallurgy, fire-resistant product for the construction industry.
Magnesium oxide is also used as a raw material for the preparation of salts such as magnesium nitrate and sulfate. It is also used for the industrial production of magnesium. It is then reduced in an electric furnace at 1100°C in the presence of silicon according to the reaction:
2MgO(s) + Si(l) → 2Mg(g) + SiO2(l).
Magnesium oxide also finds therapeutic use as an antacid, to calm heartburn. In food it is used as a food additive and regulated under number E530. It is an anti-caking agent.
Buy magnesium oxide?
You can buy pharmaceutical grade magnesium oxide at Laboratoriumdiscounter. European quality magnesium oxide. Delivered quickly and always with volume discount!
Technical data:
Magnesia burns
Empirical formula MgO
Molar mass (M) 40.30 g/mol
Density (D) 3.58 g/cm³
Boiling point (bp) 3600 °C
Melting point (mp) ~2800 °C
WCK 1
CAS no. [1309-48-4]
EC-No. 215-171-9
Downloads