You have no items in your shopping cart
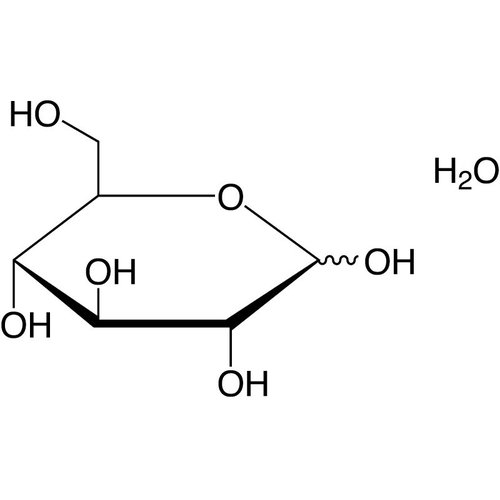
D(+)-Glucose monohydrate 99,5+% Foodgrade
- Buy 2 and save 5%
- Buy 6 and save 10%
Glucose is a sugar with the molecular formula C6H12O6. The word "glucose" comes from the ancient Greek τὸ γλεῦκος / gleukos which denoted sweet or viscous wines, or even musts. The suffix -ose is a chemical classification indicating that it is a carbohydrate. Because it cannot be hydrolyzed into simpler carbohydrates, it is an ose or monosaccharide. The presence of an aldehyde functional carbonyl group in its linear form makes it an aldose while the six carbon atoms make it a hexose; it is therefore an aldohexose.
The D-isomer, also called dextrose, is common in the natural environment, while the L-isomer is very rare there. L-glucose and D-glucose are two of the 16 stereoisomers of aldohexoses. In particular, D-glucose is synthesized by many organisms from water and carbon dioxide in the presence of light energy through photosynthesis. Conversely, the breakdown of glucose, which releases carbon dioxide and water during cell respiration, is a very important source of energy for living cells.
Glucose is stored in plants in the form of starch and in animals in the form of glycogen, which can be hydrolyzed at any time to return glucose molecules ready to be broken down by providing energy as soon as the cell needs it. It also plays a structural role as cellulose in plants. Glucose circulates throughout the body in the blood and the concentration of glucose in the blood plasma is called blood sugar.
Concentrated glucose solutions (> 30%) crystallize spontaneously at room temperature. Glucose in crystallized form is sold as dextrose to avoid confusion with corn syrups, and is in monohydrate form. Dextrose solutions are produced industrially by total hydrolysis of starch paste. Hydrolysis is performed hot by enzymatic systems combining - and β-amylases.
Glucose does not react in the presence of a dilute acid: it is a monosaccharide and is therefore not hydrolysable. In a concentrated and hot acidic medium, glucose undergoes dehydration.
In a dilute alkaline medium, for example in the presence of sodium hydroxide NaOH (sodium hydroxide), glucose forms an unstable enediol which produces:
fructose by isomerization;
mannose by epimerization.
Why buy Glucose at Laboratoriumdiscounter?
Glucose at Laboratory Discounter is of the highest quality and certified food grade. Because it is produced in Europe, quality control is high and you are guaranteed high quality Glucose. In addition, Laboratoriumdiscounter offers glucose for a friendly price. So you get the Best Glucose for the best Price!
Technical information:
Molar mass (M) 198,17 g/mol
Melting point (mp) 83 °C
WGK 1
CAS No. [77938-63-7]
EG-Nr. 200-075-1