You have no items in your shopping cart
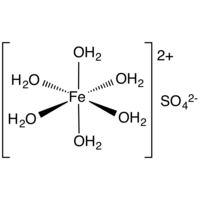
Iron (II) sulfate heptahydrate
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Iron(II) Sulphate?
Iron (II) sulfate (also ferrous sulfate, green salt, iron vitriol, formerly also green Galitzenstein) is a divalent iron salt of sulfuric acid. The name green salt for iron (II) sulfate heptahydrate (FeSO4 7 H2O) is derived from the greenish color of the salt containing water of crystallization.
What is Iron Sulphate used for?
Iron sulfate is used for coagulation water treatment and to remove phosphates in municipal and industrial sewage treatment plants to prevent eutrophication of surface water bodies.
Large amounts of this salt can be used as a reducing agent, especially for the reduction of chromates in cement.
Iron sulfate is used in the replacement of inks, called iron gall ink, which was used from the Middle Ages to the American Revolution. It is also used to dye wool as a mordant.
In 18th century England, two different methods were used for the direct application of indigo dye, and were used into the 19th century. One of these methods, known as China Blue, uses ferrous sulfate. After printing an insoluble form of indigo onto the fabric, the indigo is calculated to leucoindigo in a series of iron sulfate baths (with re-oxidation to indigo by air, between immersions).
Iron sulfate can also be used to give concrete a rusty yellowish color.
Carpenters use iron sulfate solutions to give maple wood a silvery hue.
In horticulture it is used as a lawn improver and to remove mosses.
In the second half of the 19th century, iron sulfate was used as a photographic image developer by the wet collodion processes.
Iron sulfate is sometimes added to cooling water passing through the copper tubes of a turbine condenser to prevent erosion and to protect the coating on the inside of these tubes.
In mycology it is used to recognize some mushrooms, so the Russula cyanoxantha distinguishes itself within the russulas from the other russulas that do react with iron sulphate.
Buy iron sulfate?
You will find inexpensive iron sulphate at Laboratorium discounter. Not only very competitively priced but also of high quality! Always with graduated discount and available in different packaging.
What Safety Precautions Should I Take Before Using Chemicals?
It is always wise to work as safely as possible when working with chemicals. Even substances that do not seem dangerous at first can cause enormous damage if they get into your eyes. Therefore, always wear safety goggles. You also want to prevent chemicals from ending up on your skin, which is why it is important to always use good gloves or disposable gloves.
Respiratory protection is necessary for volatile substances, vaporous liquids and solids that dust. There are many different types of filters, so you should always refer to the MSDS to find out which filter you need. Many filters also specify which substances they are for.
In some cases it may be necessary to protect the whole body, in which case a plastic overall is needed, you can find it here.
When working with flammable and oxidizing substances, it is important to always have fire extinguishers and absorbents at hand. It is also useful to be able to clean up spills of aggressive chemicals immediately with absorbents and to dispose of them in accordance with international and/or local legislation.
If you need more information on how to handle a specific substance, always consult the safety data sheet. You can find this on the product page or request it via [email protected]
Technical data:
Empirical formula FeSO4 7 H2O
Molar mass (M) 278.02 g / mol
Density (D) 1.89
Melting point (mp)> 60 ° C
Solubility approx. 400 g / l (H2O, 20 ° C)
WGK 1
CAS No. [7782-63-0]
EC No. 231-753-5
$$$$$
Hazard statements
H302 Harmful if swallowed
H315 Causes skin irritation
H319 Causes serious eye irritation
Precautions - prevention
P280 Wear protective gloves / eye protection.
Precautions - response
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P332 + P313 In case of skin irritation: Get medical advice / attention.

%%%%%
| MSDS Ijzer(II)sulfaat (NL) |
| MSDS Eisen(II)sulfat (DE) |
| MSDS Iron(II)sulfate (EN) |
| MSDS Sulfate de fer (II) (FR) |
| MSDS Sulfato de hierro (II) (ES) |