You have no items in your shopping cart
Iron(III) chloride solution 40 %
- Buy 2 and save 5%
- Buy 10 and save 10%
What is Iron(III) Chloride?
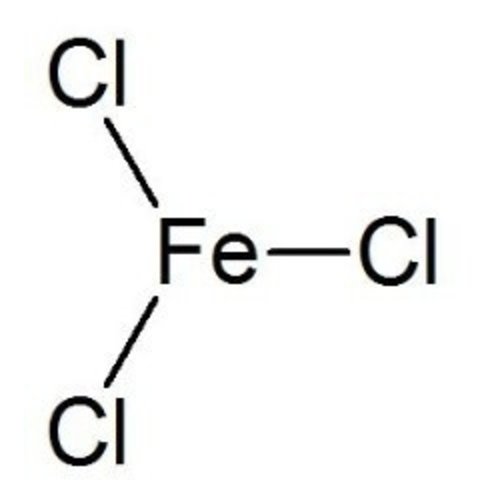
Iron(III) chloride is a chemical compound of iron(III) and chloride ions. The Roman numeral III indicates the oxidation number of the iron ion (in this case +3). Iron(III) chloride belongs to the group of iron halides.
The term ferric chloride also includes the compound iron(II) chloride (FeCl2)
What is ferric chloride used for?
Iron(III) chloride can oxidize and dissolve copper; Therefore, you can use aqueous ferric chloride solutions for gentle etching of printed circuit boards.
Iron(III) chloride is used to bind hydrogen sulfide, for phosphate precipitation and also as a precipitating agent in co-precipitation and generally as a flocculant in biological wastewater treatment. In the chemical industry, it is used as a selective catalyst in many Friedel-Crafts reactions. Many phenols produce green or blue colored complexes with iron(III) chloride and can be detected in this way (ferric chloride test). The Berlin blue pigment can be produced by adding potassium hexacyanoferrate(II).
In aqueous solution it is used in textile printing as an oxidizing agent and color mordant, in medicine for intravenous substitution in severe deficiencies and as a hemostatic agent (hemotyptic or astringent, no longer available in Germany), for corrosion tests (according to ASTM G48A) and for etching of metals (e.g. in copper intaglio printing) and of printed circuit boards in printed circuits and in the manufacture of dyes (e.g. aniline black).
Buy ferric chloride?
The ferric chloride solution from Laboratory Discounter is of the highest quality and competitively priced! Packed in sturdy packaging and always with volume discount! Delivered quickly and available in different packaging!
What Safety Precautions Should I Take Before Using Chemicals?
It is always wise to work as safely as possible when working with chemicals. Even substances that do not seem dangerous at first can cause enormous damage if they get into your eyes. Therefore, always wear safety goggles. You also want to prevent chemicals from ending up on your skin, which is why it is important to always use good gloves or disposable gloves.
Respiratory protection is necessary for volatile substances, vaporous liquids and solids that dust. There are many different types of filters, so you should always refer to the MSDS to find out which filter you need. Many filters also specify which substances they are for.
In some cases it may be necessary to protect the whole body, in which case a plastic overall is needed, you can find it here.
When working with flammable and oxidizing substances, it is important to always have fire extinguishers and absorbents at hand. It is also useful to be able to clean up spills of aggressive chemicals immediately with absorbents and to dispose of them in accordance with international and/or local legislation.
If you need more information on how to handle a specific substance, always consult the safety data sheet. You can find this on the product page or request it via [email protected]
ADR 8, III • WGK 1
CAS No.[7705-08-0]
UN-Nr. 2582
$$$$$
Hazard statements
H290 May be corrosive to metals
H302 Harmful if swallowed
H315 Causes skin irritation
H317 May cause an allergic skin reaction
H318 Causes serious eye damage
Precautions - prevention
P280 Wear protective gloves / eye protection.
Precautions - response
P302 + P352 IF ON SKIN: Wash with plenty of water.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P310 Immediately call a POISON CENTER / doctor.


%%%%%
| MSDS Ijzer(III)chloride 40% (NL) |
| MSDS Eisen(III)-chlorid 40% (DE) |
| MSDS Iron(III)chloride 40% (EN) |
| MSDS Chlorure de fer (III) 40% (FR) |
| MSDS Cloruro de hierro (III) 40% (ES) |