You have no items in your shopping cart
Maleic Anhydride 99.5+% Extra Pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Maleic Anhydride?
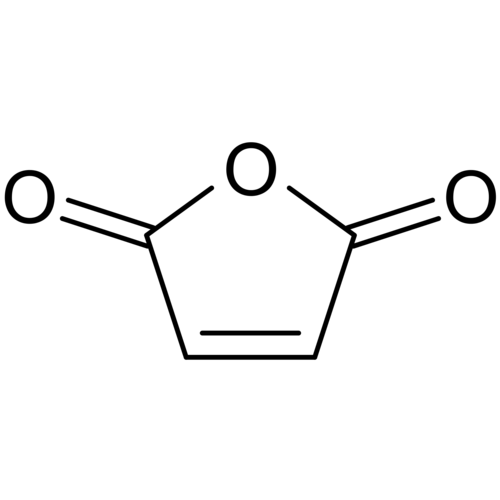
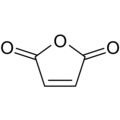
Maleic anhydride (cis-butenedioic anhydride, toxilic anhydride, 2,5-dioxofuran) is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid and when pure is a colorless or white solid with a pungent odor.
Maleic anhydride was traditionally produced by the oxidation of benzene or other aromatic compounds. As of 2006, only a few smaller factories still use benzene, due to the rising price of benzene, most maleic anhydride factories now use n-butane as raw material.
What is Maleic Anhydride used for?
Maleic anhydride is a suitable raw material for the production of many other substances. This is due to the reactivity of the double bond in combination with the two carbonyl groups.
Although maleic anhydride can polymerize itself, due to the presence of the double bond, it is generally used in copolymers. The main industrial application of maleic anhydride is polymerization with glycols and linear unsaturated epoxides to form polyesters. A third monomer, such as styrene or vinyl chloride, is generally used to provide a strong, rigid, and insoluble three-dimensional macromolecule. It is a technical polymer, which is used with fiber reinforcement for, among other things, certain parts of the interior equipment of vehicles, such as the instrument panel or the console between the front seats. Glass fibers reinforced with unsaturated polyesters are used in shipbuilding, construction, bathroom components, plastic tanks and pipes, etc.
Maleic anhydride is also used for paper treatment and in floor wax. Copolymers with acrylic acid are used in detergents.
Maleic anhydride derivatives are also used as a lubricant additive.
Maleic hydrazide is used commercially in agriculture and horticulture as a plant growth regulator. It has the property of preventing the growth of plants without having to kill them. Maleic anhydride is also used for the synthesis of various pesticides (insecticides and fungicides, herbicides, plant growth regulators), such as malathion (defunct), daminozide (trade name Alar, deprecated), captan, and endothal.
Cycloaddition with 1,3-butadiene produces tetrahydrophthalic acid. Maleic anhydride hydrolyzes in water at room temperature to maleic acid. At higher temperatures, hydrolysis results in fumaric acid (a stereoisomer of maleic acid). Fumaric acid is used in the paper industry, but also to acidify foods such as malic acid.
Buy maleic anhydride?
You can buy maleic anhydride at Laboratoriumdiscounter, not only because it is of the highest quality, but also because it is offered for a low price. Available in different packaging and delivered quickly! So order your Maleic Anhydride at Laboratoriumdiscounter.nl!
Technical data
Molar mass (M) 98,06 g/mol
Density (D) 1,48 g/cm³
Boiling point (bp) 200,1 °C
Flash point (flp) 103 °C
Melting point (mp) 53 °C
ADR 8 III
WGK 1
CAS No. 108-31-6
EG-Nr. 203-571-6
UN-Nr. 2215
Downloads
Hazard statements
H302 Harmful if swallowed
H314 Causes severe skin burns and eye damage
H317 May cause an allergic skin reaction
H334 May cause allergy or asthma symptoms or breathing difficulties if inhaled
H372 Causes damage to organs (respiratory system) through prolonged or repeated
exposure (if inhaled)
Precautionary statements
Precautionary statements - prevention
P280 Wear protective gloves/protective clothing/eye protection/face protection
Precautionary statements - response
P301+P330+P331 IF SWALLOWED: rinse mouth. Do NOT induce vomiting
P302+P352 IF ON SKIN: Wash with plenty of soap and water
P304+P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing
P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact
lenses, if present and easy to do. Continue rinsing
P310 Immediately call a POISON CENTER/doctor
Supplemental hazard information
EUH071 Corrosive to the respiratory tract.