You have no items in your shopping cart
Phosphoric acid 75%, pure, food grade
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Phosphoric Acid?
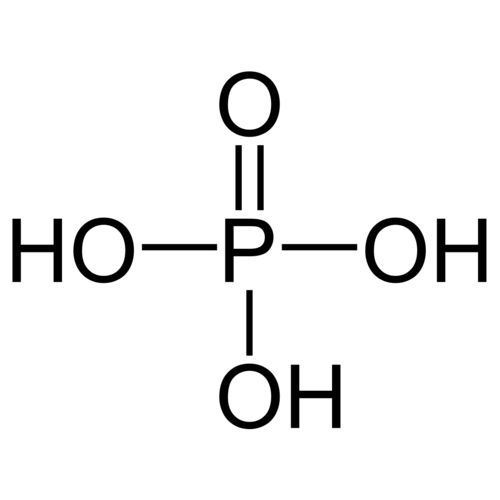
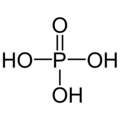
Phosphoric acid or orthophosphoric acid is the main acid of phosphorus and one of the most important inorganic acids. It is a tri-protonic acid and reacts as a moderately strong acid with respect to the first deprotonation. Their salts and esters are called phosphates, the term organophosphate is also common for esters of phosphoric acid. The phosphorus in these compounds has the oxidation state V. Thomas Graham has done important work to clarify the structure. The condensates diphosphoric acid, meta- and polyphosphoric acid are also derived from phosphoric acid. As a food additive, phosphoric acid is indicated as E 338.
What is phosphoric acid used for?
Phosphoric acid is used as a feedstock for the production of fertilizers containing phosphate, detergents, rust removers and rust converters and for the passivation of iron and zinc to protect against corrosion.
It acts as a rust converter when applied directly to rusty iron, steel and other metal surfaces. It converts red-brown iron, i.e. iron(III) oxide, into black iron(III) phosphate. After this treatment, the black iron phosphate coating can be easily washed off, revealing the fresh metal surface underneath.
It is used to produce buffer solutions.
Phosphoric acid is corrosive in high concentrations; diluted it is used in the food industry as a preservative, acidity regulator, acidity regulator and antioxidant (to prevent fats from going rancid and discolouring, for example in meat, sausage or cake fillings) (E 338) ] Apart from its corrosive effect, phosphoric acid is not toxic to the human organism.
It is used as an acidity regulator for foods and drinks, such as cola. However, this particular use of phosphoric acid has generated controversies that have raised many questions about its possible health effects on the human body.
In dentistry, phosphoric acid is combined with zinc powder to form zinc phosphate, which is used as a temporary dental cement. It is also used in orthodontics as a caustic solution to clean and roughen the surface of teeth before installing braces and other dental appliances. It is also used in many teeth whitening solutions to remove plaque that may be present on the surface of the teeth.
It is used as an electrolyte in phosphoric acid fuel cells. It is also used as a cleaner in the construction industry to remove mineral deposits, cement stains and hard water.
Hot phosphoric acid is used in microelectronics to etch silicon nitride. It is used by hobbyists as a flux to aid the soldering process. It is also used in hydroponic pH solutions to lower the pH of nutrient solutions.
Phosphoric acid is also used as an electrolyte in copper electropolishing for deburring. It is often used as a wet etchant in compound semiconductor processing.
Buy phosphoric acid?
you will find pure phosphoric acid at Laboratoriumdiscounter.nl Not only the highest quality, but also food grade certified and that for a friendly price. Delivered quickly and available in different packaging! Always with volume discount.
Technical data
Orthophosphoric acid
Empirical formula H3PO4
Molar mass (M) 98.00 g / mol
Density (D) 1.58 g / cm³
Boiling point (bp) 135 ° C
Melting point (mp) -20 ° C
ADR 8 III
WGK 1
CAS No. [7664-38-2]
EC no. 231-633-2
UN No. 1805
Downloads
$$$$$
Hazard statements
H290 May be corrosive to metals
H314 Causes severe burns and eye damage
Precautions - prevention
P280 Wear protective gloves / protective clothing / eye protection / face protection.
Precautions - response
P301 + P330 + P331 IF SWALLOWED: rinse mouth. DO NOT induce vomiting.
P303 + P361 + P353 IF ON SKIN (or hair): Immediately contaminate clothing
pull out. Rinse skin with water [or shower].
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P310 Immediately call a POISON CENTER / doctor.