You have no items in your shopping cart
Oxalic acid 99+% Pure
- Buy 2 and save 5%
- Buy 8 and save 10%
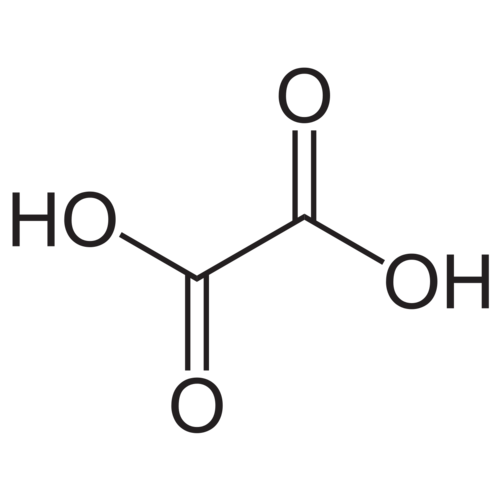
Oxalic acid or acidic acid (IUPAC name: ethanedioic acid) is a dicarboxylic acid in which both carboxy groups are directly linked to each other. The pure anhydrous substance occurs as a white crystalline powder, which is sparingly soluble in water. Oxalic acid forms a dihydrate, which is colorless, by crystallization in aqueous solutions with two molecules of crystal water.
Household
Oxalic acid can also be found in household products and anti-rust agents. A 1:10 solution is used to remove it from wood and is available for sale under the name 'design water'.
Analytical Chemistry
In analytical chemistry, oxalic acid (as a dihydrate) is used as a basic titre for basic solutions such as sodium hydroxide and potassium hydroxide, but also solutions of oxidizers such as potassium permanganate, potassium periodate or potassium dichromate. Usually, the standard concentration is about 0.1 mol / L.
Clinical chemistry
In clinical chemistry, oxalic acid is used to prevent blood from clotting. The calcium ion plays a key role in blood clotting. By binding the calcium with oxalate, it is no longer available for the clotting reaction and the blood remains liquid.
Technical product information:
MW: 90.04 g / mol
Boiling Pt: 365 ° C (1013 hPa)
Melting point: 99 to 100 ° C
Density: 1.9 g / cm³ (20 ° C)
CAS number: 6153-56-6
Downloads
$$$$$
Health and Safety
Hazard statement code (H code)
H302 + H312 H318
Harmful if swallowed and in contact with skin. Causes serious eye damage.
Preventive code (P code)
P280 P305 + P351 + P338
Wear protective gloves / eye protection / face protection.
IF IN EYES: Rinse cautiously with water for several minutes. remove contact lenses, if possible; keep rinsing.
%%%%%
| MSDS Oxaalzuur dihydraat (NL) |
| MSDS Oxalsäure dihydrat (DE) |
| MSDS Oxalic acid (EN) |
| MSDS Acide oxalique dihydraté (FR) |
| MSDS Ácido oxálico dihidrato (ES) |