You have no items in your shopping cart
Strontium Carbonate 98.5+% Pure
What is Strontium Carbonate?
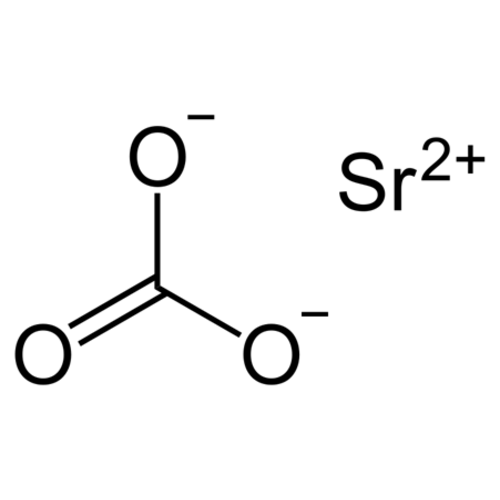
Strontium carbonate (SrCO3) is the carbonate salt of strontium. It appears as an odorless white to gray powder. It is naturally present in the form of a mineral, strontianite.
Strontium carbonate is a white, odorless, tasteless powder. It has similar properties to calcium carbonate. Being a carbonate, it is a weak base and reacts with acids. It is otherwise stable and does not pose a major risk.
It is practically insoluble in water (one part in 100,000). Its solubility increases significantly to about one part per thousand when the water is saturated with carbon dioxide, forming strontium hydrogen carbonate:
SrCO3 + H2O + CO2 → Sr(HCO3)2
It is soluble in mineral acids, due to the formation of soluble strontium salts and carbonic acid, which then itself decomposes into water and carbon dioxide:
SrCO3 + 2 HCl → SrCl2 + H2CO3
At ambient pressure, above 1200°C, strontium carbonate decomposes into strontium oxide and carbon dioxide:
SrCO3 → SrO + CO2
When the pressure is increased to 6 MPa, the strontium carbonate melts at 1497°C
What is Strontium Carbonate used for?
-Color applications
Strontium carbonate is mainly used as an inexpensive pyrotechnic dye. Strontium and its salts give off a brilliant red color in the flame. Strontium carbonate is generally preferred for its low cost and is non-hygroscopic. The ability to neutralize acids is also useful in pyrotechnics. Another similar application is its use in torches.
It is used in the manufacture of iridescent glass, luminous paints, and in the refining of sugar and certain medicines. It is also widely used in the ceramic industry as an ingredient in glazes. It acts as a flux and changes the color of certain metal oxides. It has similar properties to barium carbonate.
-Electrical/electronic applications
Strontium carbonate is used in the synthesis of strontium ferrites for the manufacture of permanent magnets used in loudspeakers and magnetic doors.
It is used in electronics, particularly in the manufacture of color cathode ray screens, for the purpose of absorbing the X-rays produced.
It is also used in the manufacture of superconductors such as BSCCO and also for electroluminescent materials where it is first calcined to SrO and then mixed with sulfur to form SrS:x, where x is generally europium. This is the famous "blue/green" phosphor that is sensitive to frequency and changes from lime green to blue when it varies from 200 to 1400 Hz. Other dopants such as gallium or yttrium can be used to obtain a yellow/orange color.
Chemistry
Due to its weak Lewis base character, strontium carbonate can be used to produce various strontium compounds by reaction with the corresponding acid.
In organic synthesis strontium carbonate can be used in combination with dihydrogen and palladium for selective reduction of double bonds.
Technical data:
Empirical formula SrCO3
Molar mass (M) 147,63 g/mol
Density (D) 3,7 g/cm³
Melting point (mp) 1200 °C
CAS No. [1633-05-2]
EG-Nr. 216-643-7
Downloads