You have no items in your shopping cart
Tin (IV) oxide 99.7 +% P.a.
- Buy 2 and save 5%
- Buy 10 and save 10%
What is Tin(IV) oxide
Tin (IV) oxide, also known as tin dioxide, is the main component of the mineral cassiterite (tin stone) and the main source of pure tin.
Tin(IV) oxide can be obtained by burning tin in air, reacting tin(IV chloride and water (both as steam) at high temperatures, and reacting tin with sulfuric acid and then with hydroxides.
What is Tin Oxide used for?
In photovoltaic cells, tin(IV) oxide is used as a transparent, electrically conductive electrode or layer, for example in the Grätzel cell. Because pure tin(IV)oxide has poor electrical conductivity, it is added (doped) with antimony (antimony-doped tin oxide, ATO) or fluorine (fluorine-doped tin oxide, FTO). Or is itself the dopant as in indium tin oxide ( ITO). Tin dioxide is also used in optical fibers or LC displays - here it serves as a transparent, electrically conductive layer - and in gas sensors, where it reacts with a change in resistance to any oxidizable gas or vapor.
It is also used as a polishing powder for steel, glass and natural stone, as a white, transparent opacifier in the production of ceramic glazes, frosted glass and enamels, for sealing cracks in glass and as a catalyst in chemical processes. Tin(IV) oxide was formerly used as a glaze in the manufacture of faience. It is also used as an electrode material in inorganic glass melting. It is mainly used as a substitute for molybdenum electrodes in lead glass melting.
In electromechanical switchgear, such as B. Protect, the contact material is often silver with 2–14 wt% SnO2. Mainly because many manufacturers have avoided cadmium, the previously used cadmium oxide has been replaced by tin oxide.
Buy tin oxide?
Do you need high quality Tin Oxide but don't want to pay too much? Then you have come to the right place at Laboratoriumdiscounter.nl. This Tin (IV) oxide is produced in Europe and of very high quality and for a friendly price! Delivered quickly.
Technical data
Stannic oxide, Flowers of tin, Tin dioxide
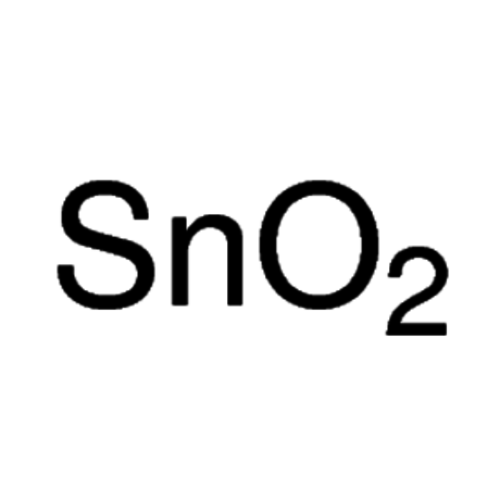
Empirical formula SnO2
Molar mass (M) 150,71 g/mol
Density (D) 6,95 g/cm³
Boiling point (bp) 1900 °C
Melting point (mp) 1630 °C
CAS No. 18282-10-5
EG-Nr. 242-159-0
Downloads