You have no items in your shopping cart
Toluene 99.9 +%, extra pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Toluene?
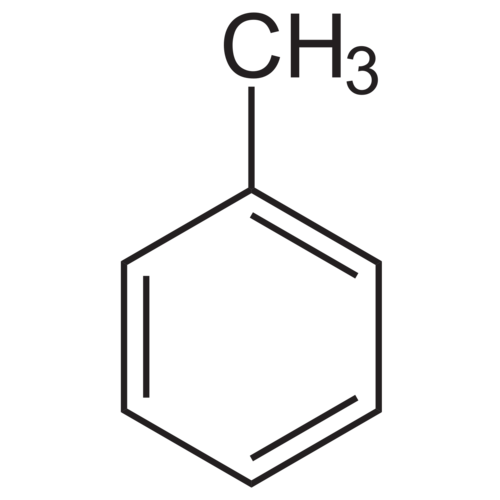
Toluene, also called methylbenzene or phenylmethane, is an aromatic hydrocarbon. It is often used as a reagent or solvent, especially in the industrial environment. It dissolves a large number of oils, fats or resins (natural or synthetic). Under normal conditions it is a transparent liquid with a characteristic odor reminiscent of paint remover or related benzene.
The compound was first isolated in 1837 by distillation of pine oil by Polish chemist Philippe Walter (in) who christened it "retinnapht" 20.21. In 1841, French chemist Henri Sainte-Claire Deville isolated it from Tolu's balm—an aromatic extract of the tropical Colombian tree Myroxylon balsamum—which Deville identified with Walter's retin naphtha and benzene; he then calls this new hydrocarbon "benzoene" 22, 23, 24. In 1843 Jöns Jacob Berzelius recommended the name "tolum" 25 . In 1850, French chemist Auguste Cahours isolated a hydrocarbon from a wood distillate that he recognized as identical to Deville's "benzoen" which he christened "toluene".
Toluene is a liquid under normal temperature and pressure conditions. It is almost insoluble in water (0.535 µl - 1 at 25 °C), but miscible with many organic solvents (acetone, diethyl ether, chloroform, ethanol, etc.), and soluble in glacial acetic acid. .
Toluene has a cryoscopic molal constant of 3.55 °C kg mol - 1 and an ebullioscopic molal constant of 3.40 °C kg mol - 1. The vapor is heavier than air and forms an explosive mixture with it.
In proton NMR, toluene appears in the form of several peaks between 7.28 ppm and 7 ppm for the hydrogen atoms of the benzene ring and of a singlet at 2.38 ppm for the hydrogen atoms of the methyl group [ref. necessary]. In carbon NMR, toluene is presented under several peaks, at 137.8 ppm for the α-carbon of methyl, at 129.09 ppm for the β-carbons, at 128.28 ppm for the γ-carbons and at 125.38 ppm for the carbon opposite to methyl. The carbon atom of the methyl group shows a peak at 25 ppm [ref. necessary].
The thermal conductivity (in W m - 1 K - 1) is 0.1425 - 0.00025T, with T expressed in °C
What is Toluene used for?
to increase the octane number in fuels mixed with benzene and xylenes. It is therefore present in various petroleum fuels;
as an extraction solvent in the cosmetics industry (perfume) and in the pharmacochemical industry;
as a solvent or ingredient in the manufacture of paints, varnishes, lacquers, waxes and inks (printing, etc.);
raw material for various industrial processes: synthesis of rubber, phenol, toluene diisocyanate (TDI), necessary to obtain the foam of polyurethane, benzene and xylenes, nitrotoluene, benzyl chloride, benzaldehyde, p-acid toluene sulfonic acid, vinyl toluene, etc.;
in the manufacture of adhesives and glues (no longer allowed);
leather tanning;
as a booster for the surfaces of certain table tennis players (no longer allowed).
Technical data
Methylbenzene
Empirical formula C7H8
Molar mass (M) 92,14 g/mol
Density (D) 0,87 g/cm³
Boiling point (bp) 110,6 °C
Flash point (flp) 4,4 °C
Melting point (mp) -95 °C
ADR 3 II
WGK 3
CAS No. 108-88-3
EG-Nr. 203-625-9
UN-Nr. 1294
Downloads
$$$$$
Hazard statements
H225 Highly flammable liquid and vapor
H304 May be fatal if swallowed and enters airways
H315 Causes skin irritation
H336 May cause drowsiness or dizziness
H361d Suspected of damaging the unborn child
H373 May cause damage to organs through prolonged or repeated exposure
Precautions - prevention
P210 Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. Do not smoke.
P280 Wear protective gloves / protective clothing / eye protection / face protection.
Precautions - response
P302 + P352 IF ON SKIN: Wash with plenty of soap and water.
P308 + P313 NA (possible) exposure: Get medical attention.
P331 DO NOT induce vomiting.