You have no items in your shopping cart
Urea ≥99,5 %, pure
- Buy 2 and save 5%
What is Urea?
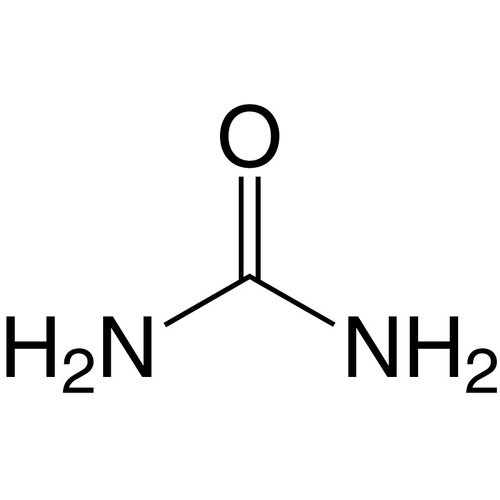
Urea (from Greek ouron, in turn from Indo-European awer, "moisture, current") is a chemical compound with the formula CO(NH2)2. It is found to a greater extent in urine, sweat and feces. It is the major end product of protein metabolism in mammals, such as humans. Human urine contains about 20 g/L (grams per litre); an adult eliminates 25 to 39 g (grams) daily. It is one of the few organic compounds that does not have C-C or C-H bonds.
In smaller amounts it occurs in the blood, liver, lymph and serous fluids, as well as in the faeces of fish and many other animals. It is also found in the heart, lungs, bones and reproductive organs, as well as semen. Urea is mainly formed in the liver as an end product of metabolism. Urea nitrogen, which makes up 80% of the nitrogen in urine, comes from the breakdown of various nitrogenous compounds, especially amino acids from protein in food. In mammals, urea is formed in a metabolic cycle called the urea cycle. Urea is also present in fungi and in the leaves and seeds of numerous legumes and grains.
Due to the dipole moment, urea is soluble in water and alcohol and slightly soluble in ether.
What is Urea used for?
Fertilizer
91% of the urea produced is used as fertilizer. It is applied directly to the soil and supplies nitrogen to the plant. Urea with a low biuret content (less than 0.03%) is also used as a foliar fertilizer. It dissolves in water and is applied to the leaves of plants, especially fruit trees, citrus fruits.
Urea as a fertilizer has the advantage of providing a high nitrogen content, which is essential for the plant's metabolism, as it is directly related to the number of stems and leaves, which absorb light for photosynthesis. In addition, nitrogen is present in vitamins and proteins and is related to the protein content of grains.
Granulated urea is the main fertilizer that provides nitrogen to the soil to increase productivity because it has the highest concentration of nitrogen. Applies to all crops.
Urea adapts to different types of crops. It is necessary to fertilize, because a large amount of nitrogen is lost during harvesting. The grain is applied to the soil, which must be well worked and rich in bacteria. The application can be done at the time of planting or before. The grain is then hydrolyzed and broken down.
Great care must be taken in the correct application of urea to the soil. If it is applied to the surface or if it is not absorbed into the soil, either through proper application, rain or irrigation, the ammonia evaporates and the losses are very significant. The lack of nitrogen in the plant is manifested in a decrease in leaf area and a decrease in photosynthetic activity.
To determine the correct dose per crop, diagnostic tools are often used to achieve the highest yield in a sustainable way. In the case of maize, tools such as Maicero are used to evaluate the productive behavior of the crop with increasing nitrogen fertilization doses, taking into account management, soil and climate scenarios of the growing region. This tool was developed by Profertil in collaboration with CREA and the Faculty of Agronomy Argentina.
Chemical and plastic industry.
It is present in adhesives, plastics, resins, inks, pharmaceuticals and finishes for textiles, paper, metals and tobacco. Such as urea formaldehyde resin. These resins have various applications in industry, such as the production of chipboard. It is also used in the production of cosmetics and paint.
Feed supplement for cattle
It is mixed in animal feed and provides nitrogen, essential for the formation of proteins.
Part of the additive Adblue or urea AUS32
an additive used to reduce nitrogen oxide (NOx) emissions caused by diesel engine exhaust through a process called selective catalytic reduction (RCA).
Cosmetic industry
Urea is used in cosmetic formulations for its moisturizing properties (5-20%) and its exfoliating or keratolytic properties (30-50%). Helps remove dead cells and calluses. In the skin, it interacts with the keratin protein, breaking the hydrogen bond interactions that stabilize the secondary structure.
buy urea?
You can buy high-quality urea at Laboratoriumdiscounter.nl. Delivered quickly and available in different packaging. Always with volume discount and for a friendly price!
Technical properties
Empirical formula CH4N2O
Molar mass (M) 60,06 g/mol
Density (D) 1,32
Melting point (mp)132-135 °C
Solubility 1080 g/l (H2O, 20 °C)
WGK 1
CAS No.[57-13-6]
EG-Nr. 200-315-5
Downloads