You have no items in your shopping cart
Zinc acetate dihydrate ≥99 %, extra pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Zinc Acetate?
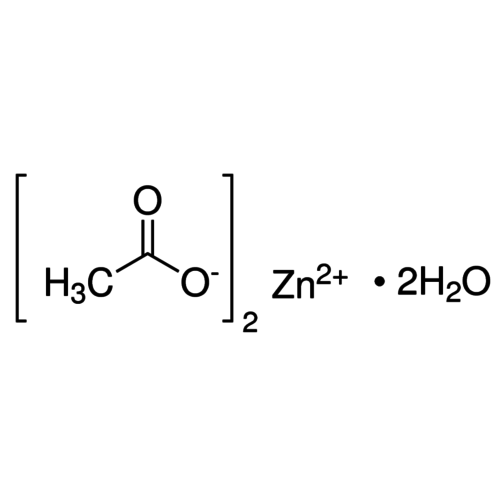
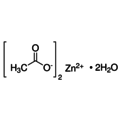
Zinc acetate is the chemical compound with the formula Zn(O2CCH3)2, which is usually produced as the dihydrate Zn(O2CCH3)2(H2O)2. Both the hydrated and anhydrous forms are colorless solids commonly used in chemical synthesis and as nutritional supplements. Zinc acetates are prepared by the action of acetic acid on zinc carbonate or on metallic zinc.
What is Zinc Acetate used for?
Industrial applications include wood preservation, manufacture of other zinc salts or polymers, manufacture of ethylene acetate, as a mordant dye and as an analytical reagent.
Zinc acetate is a sol-gel precursor of the transparent zinc oxide semiconductors.
Zinc acetate is also used in some nuclear power plants for primary circuit protection. Zinc creates a protective layer on the primary circuit that improves system chemistry.
Buy zinc acetate?
You will find zinc acetate of the best quality at Laboratoriumdiscounter.nl. Fast delivery and available in different packaging. Always with volume discount and a friendly price.
Technical information
Empirical formula Zn(CH3COO)2 · 2 H2O
Molar mass (M) 219,49 g/mol
Density (D) 1,74
Melting point (mp)237 °C
Solubility 430 g/l (H2O, 20 °C)
ADR 9 III • WGK 3
CAS No.[5970-45-6]
EG-Nr. 209-170-2 • UN-Nr. 3077
Downloads
$$$$$
Hazard statements
H302 Harmful if swallowed
H318 Causes serious eye damage
H411 Toxic to aquatic life with long lasting effects
Precautions - prevention
P273 Avoid release to the environment.
P280 Wear protective gloves / eye protection.
Precautions - response
P301 + P312 IF SWALLOWED: Call a POISON CENTER / doctor if you feel unwell.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.