You have no items in your shopping cart
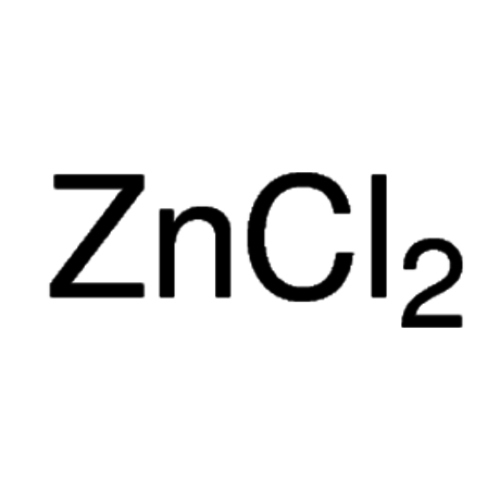
Zinc chloride≥98,5 %, pure
- Buy 2 and save 5%
- Buy 6 and save 10%
What is Zinc Chloride?
Zinc chloride (ZnCl2) is a white, granular powder that forms when zinc is heated in chlorine or zinc sulfate with calcium chloride, also when zinc, zinc oxide or zinc react with hydrochloric acid.
What is zinc chloride used for?
Zinc chloride is used to impregnate wood, preserve animal substances, refine oil, make parchment paper, vulcanized fiber, ether, stearic acid; With chlorinated lime for bleaching the paper, in dyeing as a stain for aniline blue, for rendering some tar colors and garancine, for coloring and dyeing the copper, for gluing paper pulp, for disinfection, in chemical work as a dehydrating agent , in medicine as a caustic, a concentrated solution for heating vessels evenly to a certain higher temperature.
A solution of viscous zinc chloride, mixed with zinc oxide, solidifies and gives a white, very hard mass consisting of basic zinc chloride, which can be used as a dental and metal putty, especially if you add a little glass powder. Paints that form zinc oxychloride are also recommended.
For example, 4 l of acid-free zinc chloride solution of 58 ° Bé (corresponding to a density of 1.74 g / cm3) is mixed with 10 l of a solution containing 2% soda carbonate and zinc oxide is added to the right consistency. This odorless and inexpensive mixture should be consumed immediately. The paint is permanent, but does not tolerate color additives. A solution of zinc chloride with a specific gravity of 1.7, boiled with excess zinc oxide, dissolves silk.
-Soldering fluid and soldering salt
Ammonium Zinc Chloride (NH4) 2 [ZnCl4] (Soldering Salt) crystallizes from mixed, highly concentrated solutions of zinc chloride and ammonia or from a solution of zinc oxide or zinc hydroxide in ammonia. A solution of zinc in concentrated hydrochloric acid, which contains as much ammonia as zinc (soldering water), removes the oxides from a piece of metal (copper, iron) before it is soldered or tinned.
Alternatively, an approximately 30% aqueous solution of zinc chloride, acidified with a little hydrochloric acid with the addition of a little ammonium chloride, can be used as the flux. It is used in technology as a wetting and surface activating agent for tinning at about 300 ° C. The zinc chloride can, especially in the heat on the surfaces so. B. dissolving oxides (Fe2O3) in steel by forming complexes, removing them from the steel surface and enabling direct contact between steel and tin after tinning: A strong bond is formed between steel and tin.
In smoke or smoke, zinc chloride is produced in finely divided form by burning a mixture of zinc oxide, hexachloroethane and aluminum powder. In addition to zinc chloride, the smoke produced in this way also contains hydrochloric acid and other organochlorine compounds.
Technical data
Empirical formula ZnCl2
Molar mass (M) 136.28 g / mol
Density (D) 2.91
Boiling point (bp) 732 ° C
Melting point (mp) 283 ° C
Solubility 3680 g / l (H2O, 20 ° C)
ADR 8 III • WGK 3
CAS No. [7646-85-7]
EC no. 231-592-0 • UN No. 2331
Downloads
$$$$$
Hazard statements
H302 Harmful if swallowed
H314 Causes severe burns and eye damage
H335 May cause respiratory irritation
H410 Very toxic to aquatic life with long lasting effects
Precautions - prevention
P260 Do not breathe dust.
P273 Avoid release to the environment.
P280 Wear protective gloves / eye protection.
Precautions - response
P302 + P352 IF ON SKIN: Wash with plenty of water.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for a long period of time
amount of minutes; remove contact lenses, if possible; keep rinsing.
P310 Immediately call a POISON CENTER / doctor.